当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differentiating alkyne reactivity in the post-Ugi transformations: Access to polycyclic indole-fused frameworks
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.tetlet.2018.03.079 Chao Liu , Gaigai Wang , Yingchun Wang , Kristof Van Hecke , Olga P. Pereshivko , Vsevolod A. Peshkov
中文翻译:

在Ugi后转化中区分炔烃反应性:获得多环吲哚融合的骨架
更新日期:2018-03-27
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.tetlet.2018.03.079 Chao Liu , Gaigai Wang , Yingchun Wang , Kristof Van Hecke , Olga P. Pereshivko , Vsevolod A. Peshkov
|
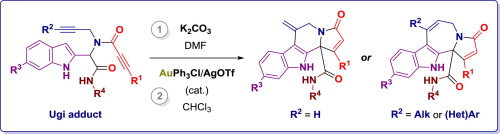
The Ugi adducts prepared from propiolic acids, propargylamines, indole-2-carbaldehydes, and isocyanides were utilized to assemble polycyclic indole-fused frameworks via two consecutive carbocyclizations involving triple bonds. First, the peptidyl position of Ugi adduct underwent potassium carbonate-mediated cyclization onto the triple bond derived from propiolic acid. Then, the position 3 of indole core engaged into gold-catalyzed cyclization onto the propargylamine-originated alkyne, completing the construction of polycyclic core.
中文翻译:

在Ugi后转化中区分炔烃反应性:获得多环吲哚融合的骨架
由丙酸,炔丙基胺,吲哚-2-甲醛和异氰酸酯制备的Ugi加合物用于通过两个连续的涉及三键的碳环化来组装多环吲哚稠合的骨架。首先,Ugi加合物的肽基位置经历了碳酸钾介导的环化作用,该环化作用源自丙酸的三键上。然后,吲哚核的位置3参与金催化的炔丙基胺来源的炔烃的环化反应,从而完成了多环核的构建。











































 京公网安备 11010802027423号
京公网安备 11010802027423号