Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-03-27 , DOI: 10.1016/j.jfluchem.2018.03.012 Helio G. Bonacorso , Alex Ketzer , Wilian C. Rosa , Tainara P. Calheiro , Melissa B. Rodrigues , Nilo Zanatta , Marcos A.P. Martins , Clarissa P. Frizzo
|
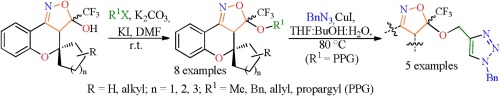
The present paper describes first an efficient methodology for an easy O-functionalization (alkylation) of tetracyclic hydroxyisoxazolines 1 applied to the synthesis of eight examples of new 3-(alkoxy)-3-(trifluoromethyl)-3,3a-dihydrospiro[chromeno [4,3-c]isoxazole-4,1′-cycloalkanes] (6-9) at 61–84% yields, in which the alkoxy substituents were methoxy, benzyloxy, allyloxy, or prop-2-yn-1-yloxy; while the cycloalkanes were 5-, 6-, and 7-membered carbocycles, as well as 4′-methyl- and 4′-t-butyl-cyclohexane. The reaction medium used 1, four alkyl halides (2-5), K2CO3, and KI in DMF at room temperature. The selected 3-(prop-2-yn-1-yloxy)-substituted isoxazolines 6 were successfully used as a CC-fragment in the construction of another series of five examples of 3-((1-benzyl-1H-1,2,3-triazol-4-yl)methoxy)-3-(trifluoromethyl)-3,3a-dihydrospiro[chromeno[4,3-c]isoxazole-4,1′-cycloalkanes] (10), at 36–84% yields, through a regioselective 1,3 dipolar cycloaddition reaction catalyzed by copper iodide (CuAAC), using benzyl azide as an NNN-building block (Click Chemistry).
中文翻译:

三氟甲基取代的螺四环异恶唑啉的O-官能化的有用方法及其在1,2,3-三唑衍生物的合成中的应用
本文首先介绍了一种有效的方法,用于四环羟基异恶唑啉1的简单O-官能化(烷基化),该方法可用于合成新的3-(烷氧基)-3-(三氟甲基)-3,3a-二氢螺基[chromeno [ 4,3 - c ]异恶唑-4,1'-环烷烃](6-9)的产率为61–84%,其中烷氧基取代基为甲氧基,苄氧基,烯丙氧基或丙-2-炔-1-基氧基;而环烷烃是5、6和7元碳环以及4'-甲基和4'-叔丁基-环己烷。将反应介质用1,四烷基卤化物(2 - 5),K 2 CO 3,以及在室温下在DMF中使用KI。所选的3-(prop-2-yn-1-yloxy)-取代的异恶唑啉6成功地用作CC片段,用于构建3-((1-苄基-1 H -1, 2,3-三唑-4-基)甲氧基)-3-(三氟甲基)-3,3a-二氢螺[铬色[4,3 - c ]异恶唑-4,1'-环烷烃](10),在36–84通过使用叠氮化苄基作为NNN结构单元(点击化学),由碘化铜(CuAAC)催化的区域选择性1,3偶极环加成反应,得到3%的收率。









































 京公网安备 11010802027423号
京公网安备 11010802027423号