当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The enhancement and suppression of immersion mode heterogeneous ice-nucleation by solutes
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-27 00:00:00 , DOI: 10.1039/c7sc05421a Thomas F Whale 1 , Mark A Holden 1, 2, 3 , Theodore W Wilson 1 , Daniel O'Sullivan 1 , Benjamin J Murray 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-27 00:00:00 , DOI: 10.1039/c7sc05421a Thomas F Whale 1 , Mark A Holden 1, 2, 3 , Theodore W Wilson 1 , Daniel O'Sullivan 1 , Benjamin J Murray 1
Affiliation
|
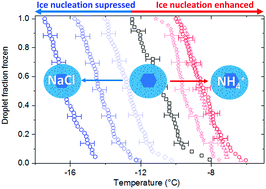
Heterogeneous nucleation of ice from aqueous solutions is an important yet poorly understood process in multiple fields, not least the atmospheric sciences where it impacts the formation and properties of clouds. In the atmosphere ice-nucleating particles are usually, if not always, mixed with soluble material. However, the impact of this soluble material on ice nucleation is poorly understood. In the atmospheric community the current paradigm for freezing under mixed phase cloud conditions is that dilute solutions will not influence heterogeneous freezing. By testing combinations of nucleators and solute molecules we have demonstrated that 0.015 M solutions (predicted melting point depression <0.1 °C) of several ammonium salts can cause suspended particles of feldspars and quartz to nucleate ice up to around 3 °C warmer than they do in pure water. In contrast, dilute solutions of certain alkali metal halides can dramatically depress freezing points for the same nucleators. At 0.015 M, solutes can enhance or deactivate the ice-nucleating ability of a microcline feldspar across a range of more than 10 °C, which corresponds to a change in active site density of more than a factor of 105. This concentration was chosen for a survey across multiple solutes–nucleant combinations since it had a minimal colligative impact on freezing and is relevant for activating cloud droplets. Other nucleators, for instance a silica gel, are unaffected by these ‘solute effects’, to within experimental uncertainty. This split in response to the presence of solutes indicates that different mechanisms of ice nucleation occur on the different nucleators or that surface modification of relevance to ice nucleation proceeds in different ways for different nucleators. These solute effects on immersion mode ice nucleation may be of importance in the atmosphere as sea salt and ammonium sulphate are common cloud condensation nuclei (CCN) for cloud droplets and are internally mixed with ice-nucleating particles in mixed-phase clouds. In addition, we propose a pathway dependence where activation of CCN at low temperatures might lead to enhanced ice formation relative to pathways where CCN activation occurs at higher temperatures prior to cooling to nucleation temperature.
中文翻译:

溶质对浸没模式异质冰核的增强和抑制
水溶液中冰的异质成核是多个领域中一个重要但人们知之甚少的过程,尤其是在影响云的形成和性质的大气科学中。在大气中,冰核颗粒通常(如果不是总是)与可溶物质混合。然而,人们对这种可溶性物质对冰成核的影响知之甚少。在大气界,当前混合相云条件下的冻结范例是稀溶液不会影响异质冻结。通过测试成核剂和溶质分子的组合,我们证明了 0.015 M 溶液(预测熔点降低 <0 id=20> 5 。选择该浓度用于跨多种溶质-成核剂组合的调查,因为它对冷冻的依数影响最小并且与激活云滴相关。其他成核剂(例如硅胶)不受这些“溶质效应”的影响,在实验不确定性范围内,这种对溶质存在的响应表明冰成核发生的不同机制。不同的成核剂或与冰成核相关的表面改性对于不同的成核剂以不同的方式进行,这些溶质对浸没模式冰成核的影响可能在大气中很重要,因为海盐和硫酸铵是云的常见云凝核(CCN)。液滴并与混合相云中的冰核颗粒内部混合。 此外,我们提出了一种途径依赖性,其中相对于在冷却到成核温度之前在较高温度下发生CCN激活的途径,低温下CCN的激活可能导致冰形成增强。
更新日期:2018-03-27
中文翻译:

溶质对浸没模式异质冰核的增强和抑制
水溶液中冰的异质成核是多个领域中一个重要但人们知之甚少的过程,尤其是在影响云的形成和性质的大气科学中。在大气中,冰核颗粒通常(如果不是总是)与可溶物质混合。然而,人们对这种可溶性物质对冰成核的影响知之甚少。在大气界,当前混合相云条件下的冻结范例是稀溶液不会影响异质冻结。通过测试成核剂和溶质分子的组合,我们证明了 0.015 M 溶液(预测熔点降低 <0 id=20> 5 。选择该浓度用于跨多种溶质-成核剂组合的调查,因为它对冷冻的依数影响最小并且与激活云滴相关。其他成核剂(例如硅胶)不受这些“溶质效应”的影响,在实验不确定性范围内,这种对溶质存在的响应表明冰成核发生的不同机制。不同的成核剂或与冰成核相关的表面改性对于不同的成核剂以不同的方式进行,这些溶质对浸没模式冰成核的影响可能在大气中很重要,因为海盐和硫酸铵是云的常见云凝核(CCN)。液滴并与混合相云中的冰核颗粒内部混合。 此外,我们提出了一种途径依赖性,其中相对于在冷却到成核温度之前在较高温度下发生CCN激活的途径,低温下CCN的激活可能导致冰形成增强。











































 京公网安备 11010802027423号
京公网安备 11010802027423号