当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Methyl phenylglycines by asymmetric α-arylation of alanine and their effect on the conformational preference of helical Aib foldamers†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1039/c8ob00551f Romain Costil 1 , Fernando Fernández-Nieto , Rachel C Atkinson , Jonathan Clayden
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1039/c8ob00551f Romain Costil 1 , Fernando Fernández-Nieto , Rachel C Atkinson , Jonathan Clayden
Affiliation
|
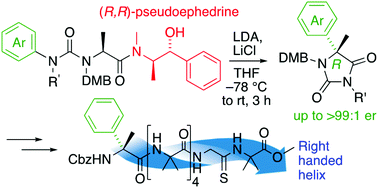
α-Arylated alanine derivatives were made enantioselectively by migratory rearrangement of a urea derivative using (R,R)-pseudoephedrine as a chiral auxiliary. Incorporation of a single residue of the product α-methyl phenylglycine into an otherwise achiral oligomer of aminoisobutyric acid oligomer induced a preferred screw sense, detectable by a NMR reporter located at the remote terminus of the oligomer. The magnitude of the screw sense induction was greater when the chiral residue was located at the N-terminus of the foldamer, and in some cases the sense of induction was opposite to that of related α-methylated amino acids with α-substituents other than aryl.
中文翻译:

α-甲基苯基甘氨酸通过丙氨酸的不对称 α-芳基化及其对螺旋 Aib 折叠分子构象偏好的影响†
α-芳基化丙氨酸衍生物是通过使用 ( R , R )-伪麻黄碱作为手性助剂对脲衍生物进行迁移重排来进行对映选择性制备的。将产物α-甲基苯基甘氨酸的单个残基掺入到氨基异丁酸低聚物的其他非手性低聚物中诱导了优选的螺旋感觉,可通过位于低聚物远端的NMR报告子检测到。当手性残基位于折叠体的 N 末端时,螺旋感诱导的幅度更大,并且在某些情况下,诱导感与具有除芳基以外的 α-取代基的相关 α-甲基化氨基酸的诱导感相反.
更新日期:2018-03-26
中文翻译:

α-甲基苯基甘氨酸通过丙氨酸的不对称 α-芳基化及其对螺旋 Aib 折叠分子构象偏好的影响†
α-芳基化丙氨酸衍生物是通过使用 ( R , R )-伪麻黄碱作为手性助剂对脲衍生物进行迁移重排来进行对映选择性制备的。将产物α-甲基苯基甘氨酸的单个残基掺入到氨基异丁酸低聚物的其他非手性低聚物中诱导了优选的螺旋感觉,可通过位于低聚物远端的NMR报告子检测到。当手性残基位于折叠体的 N 末端时,螺旋感诱导的幅度更大,并且在某些情况下,诱导感与具有除芳基以外的 α-取代基的相关 α-甲基化氨基酸的诱导感相反.











































 京公网安备 11010802027423号
京公网安备 11010802027423号