当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered Charge Redistribution of Gp2 Proteins through Guided Diversity for Improved PET Imaging of Epidermal Growth Factor Receptor
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00144 Brett A. Case 1 , Max A. Kruziki 1 , Sadie M. Johnson 1 , Benjamin J. Hackel 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-03-26 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00144 Brett A. Case 1 , Max A. Kruziki 1 , Sadie M. Johnson 1 , Benjamin J. Hackel 1
Affiliation
|
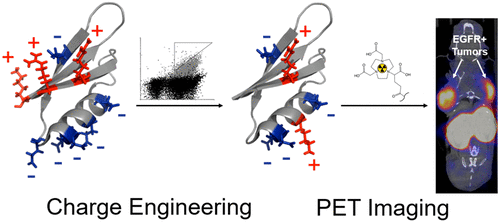
The Gp2 domain is a protein scaffold for synthetic ligand engineering. However, the native protein function results in a heterogeneous distribution of charge on the conserved surface, which may hinder further development and utility. We aim to modulate charge, without diminishing function, which is challenging in small proteins where each mutation is a significant fraction of protein structure. We constructed rationally guided combinatorial libraries with charge-neutralizing or charge-flipping mutations and sorted them, via yeast display and flow cytometry, for stability and target binding. Deep sequencing of functional variants revealed effective mutations both in clone-dependent contexts and broadly across binders to epidermal growth factor receptor (EGFR), insulin receptor, and immunoglobulin G. Functional mutants averaged 4.3 charge neutralizing mutations per domain while maintaining net negative charge. We evolved an EGFR-targeted Gp2 mutant that reduced charge density by 33%, maintained net charge, and improved charge distribution homogeneity while elevating thermal stability (Tm = 87 ± 1 °C), improving binding specificity, and maintaining affinity (Kd = 8.8 ± 0.6 nM). This molecule was conjugated with 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid for 64Cu chelation and evaluated for physiological distribution in mice with xenografted A431 (EGFRhigh) and MDA-MB-435 (EGFRlow) tumors. Excised tissue gamma counting and positron emission tomography/computed tomography imaging revealed good EGFRhigh tumor signal (4.7 ± 0.5%ID/g) at 2 h post-injection and molecular specificity evidenced by low uptake in EGFRlow tumors (0.6 ± 0.1%ID/g, significantly lower than for non-charge-modified Gp2, p = 0.01). These results provide charge mutations for an improved Gp2 framework, validate an effective approach to charge engineering, and advance performance of physiological EGFR targeting for molecular imaging.
中文翻译:

Gp2蛋白的工程电荷重分布通过引导多样性改善了表皮生长因子受体的PET成像
Gp2结构域是用于合成配体工程的蛋白质支架。但是,天然蛋白质的功能会导致保守表面上电荷的异质分布,这可能会阻碍进一步的开发和应用。我们的目标是在不降低功能的情况下调节电荷,这在小蛋白(每个突变是蛋白结构的重要组成部分)中具有挑战性。我们构建了具有电荷中和或电荷翻转突变的合理引导的组合文库,并通过酵母菌展示和流式细胞仪对它们进行了分类,以确保稳定性和靶标结合。功能变体的深度测序揭示了在克隆依赖性背景下以及广泛跨越表皮生长因子受体(EGFR),胰岛素受体和免疫球蛋白G的结合剂的有效突变。功能突变体的平均值为4。每个域3个电荷中和突变,同时保持净负电荷。我们进化出了以EGFR为目标的Gp2突变体,该突变体将电荷密度降低了33%,保持了净电荷,并改善了电荷分布的均匀性,同时提高了热稳定性(T m = 87±1°C),提高结合特异性,并保持亲和力(K d = 8.8±0.6 nM)。该分子与1,4,7-三氮杂环壬烷,1-戊二酸-4,7-乙酸缀合以进行64 Cu螯合,并评估了异种A431(EGFR high)和MDA-MB-435(EGFR)小鼠的生理分布低)肿瘤。切除的组织γ计数和正电子发射断层扫描/计算机断层扫描成像显示,注射后2 h EGFR良好的高肿瘤信号(4.7±0.5%ID / g),分子特异性由EGFR低度肿瘤的低摄取(0.6±0.1%ID)证明/ g,显着低于非电荷修饰的Gp2,p= 0.01)。这些结果为改进的Gp2框架提供了电荷突变,验证了电荷工程的有效方法,并提高了针对分子成像的生理性EGFR靶向的性能。
更新日期:2018-03-26
中文翻译:

Gp2蛋白的工程电荷重分布通过引导多样性改善了表皮生长因子受体的PET成像
Gp2结构域是用于合成配体工程的蛋白质支架。但是,天然蛋白质的功能会导致保守表面上电荷的异质分布,这可能会阻碍进一步的开发和应用。我们的目标是在不降低功能的情况下调节电荷,这在小蛋白(每个突变是蛋白结构的重要组成部分)中具有挑战性。我们构建了具有电荷中和或电荷翻转突变的合理引导的组合文库,并通过酵母菌展示和流式细胞仪对它们进行了分类,以确保稳定性和靶标结合。功能变体的深度测序揭示了在克隆依赖性背景下以及广泛跨越表皮生长因子受体(EGFR),胰岛素受体和免疫球蛋白G的结合剂的有效突变。功能突变体的平均值为4。每个域3个电荷中和突变,同时保持净负电荷。我们进化出了以EGFR为目标的Gp2突变体,该突变体将电荷密度降低了33%,保持了净电荷,并改善了电荷分布的均匀性,同时提高了热稳定性(T m = 87±1°C),提高结合特异性,并保持亲和力(K d = 8.8±0.6 nM)。该分子与1,4,7-三氮杂环壬烷,1-戊二酸-4,7-乙酸缀合以进行64 Cu螯合,并评估了异种A431(EGFR high)和MDA-MB-435(EGFR)小鼠的生理分布低)肿瘤。切除的组织γ计数和正电子发射断层扫描/计算机断层扫描成像显示,注射后2 h EGFR良好的高肿瘤信号(4.7±0.5%ID / g),分子特异性由EGFR低度肿瘤的低摄取(0.6±0.1%ID)证明/ g,显着低于非电荷修饰的Gp2,p= 0.01)。这些结果为改进的Gp2框架提供了电荷突变,验证了电荷工程的有效方法,并提高了针对分子成像的生理性EGFR靶向的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号