当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interplay between Surface Chemistry, Precursor Reactivity, and Temperature Determines Outcome of ZnS Shelling Reactions on CuInS2 Nanocrystals.
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-03-25 , DOI: 10.1021/acs.chemmater.8b00477 Anne C Berends 1 , Ward van der Stam 1 , Jan P Hofmann 2 , Eva Bladt 3 , Johannes D Meeldijk 4 , Sara Bals 3 , Celso de Mello Donega 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-03-25 , DOI: 10.1021/acs.chemmater.8b00477 Anne C Berends 1 , Ward van der Stam 1 , Jan P Hofmann 2 , Eva Bladt 3 , Johannes D Meeldijk 4 , Sara Bals 3 , Celso de Mello Donega 1
Affiliation
|
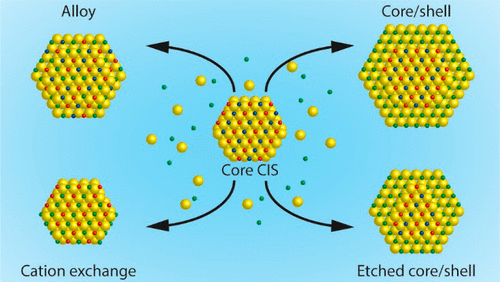
ZnS shelling of I-III-VI2 nanocrystals (NCs) invariably leads to blue-shifts in both the absorption and photoluminescence spectra. These observations imply that the outcome of ZnS shelling reactions on I-III-VI2 colloidal NCs results from a complex interplay between several processes taking place in solution, at the surface of, and within the seed NC. However, a fundamental understanding of the factors determining the balance between these different processes is still lacking. In this work, we address this need by investigating the impact of precursor reactivity, reaction temperature, and surface chemistry (due to the washing procedure) on the outcome of ZnS shelling reactions on CuInS2 NCs using a seeded growth approach. We demonstrate that low reaction temperatures (150 °C) favor etching, cation exchange, and alloying regardless of the precursors used. Heteroepitaxial shell overgrowth becomes the dominant process only if reactive S- and Zn-precursors (S-ODE/OLAM and ZnI2) and high reaction temperatures (210 °C) are used, although a certain degree of heterointerfacial alloying still occurs. Remarkably, the presence of residual acetate at the surface of CIS seed NCs washed with ethanol is shown to facilitate heteroepitaxial shell overgrowth, yielding for the first time CIS/ZnS core/shell NCs displaying red-shifted absorption spectra, in agreement with the spectral shifts expected for a type-I band alignment. The insights provided by this work pave the way toward the design of improved synthesis strategies to CIS/ZnS core/shell and alloy NCs with tailored elemental distribution profiles, allowing precise tuning of the optoelectronic properties of the resulting materials.
中文翻译:

表面化学,前体反应性和温度之间的相互作用决定了CuInS2纳米晶体上ZnS脱壳反应的结果。
I-III-VI2纳米晶体(NC)的ZnS脱壳总是导致吸收光谱和光致发光光谱发生蓝移。这些观察结果暗示,在I-III-VI2胶态NC上ZnS脱壳反应的结果是由种子NC中溶液中,溶液中和溶液中发生的多个过程之间复杂的相互作用导致的。但是,仍然缺乏对决定这些不同过程之间平衡的因素的基本了解。在这项工作中,我们通过研究前驱反应性,反应温度和表面化学(由于洗涤程序而定)对使用种子生长法在CuInS2 NC上进行ZnS脱壳反应的结果的影响,从而满足了这一需求。我们证明了低反应温度(150°C)有利于蚀刻,阳离子交换,和合金化,与使用的前体无关。仅当使用反应性S和Zn前体(S-ODE / OLAM和ZnI2)和高反应温度(210°C)时,异质外延壳的过度生长才成为主要过程,尽管仍会发生一定程度的异相合金化。值得注意的是,显示出用乙醇洗涤的CIS种子NC的表面残留乙酸盐会促进异质外延壳的过度生长,并首次使CIS / ZnS核/壳NC产生红移的吸收光谱,这与光谱偏移一致预期用于I型频段对齐。这项工作提供的见解为设计具有定制元素分布轮廓的CIS / ZnS核/壳和合金NC提供了改进的合成策略,
更新日期:2018-03-25
中文翻译:

表面化学,前体反应性和温度之间的相互作用决定了CuInS2纳米晶体上ZnS脱壳反应的结果。
I-III-VI2纳米晶体(NC)的ZnS脱壳总是导致吸收光谱和光致发光光谱发生蓝移。这些观察结果暗示,在I-III-VI2胶态NC上ZnS脱壳反应的结果是由种子NC中溶液中,溶液中和溶液中发生的多个过程之间复杂的相互作用导致的。但是,仍然缺乏对决定这些不同过程之间平衡的因素的基本了解。在这项工作中,我们通过研究前驱反应性,反应温度和表面化学(由于洗涤程序而定)对使用种子生长法在CuInS2 NC上进行ZnS脱壳反应的结果的影响,从而满足了这一需求。我们证明了低反应温度(150°C)有利于蚀刻,阳离子交换,和合金化,与使用的前体无关。仅当使用反应性S和Zn前体(S-ODE / OLAM和ZnI2)和高反应温度(210°C)时,异质外延壳的过度生长才成为主要过程,尽管仍会发生一定程度的异相合金化。值得注意的是,显示出用乙醇洗涤的CIS种子NC的表面残留乙酸盐会促进异质外延壳的过度生长,并首次使CIS / ZnS核/壳NC产生红移的吸收光谱,这与光谱偏移一致预期用于I型频段对齐。这项工作提供的见解为设计具有定制元素分布轮廓的CIS / ZnS核/壳和合金NC提供了改进的合成策略,











































 京公网安备 11010802027423号
京公网安备 11010802027423号