当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate Directed Asymmetric Reactions
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1021/acs.chemrev.7b00514 Sukalyan Bhadra 1 , Hisashi Yamamoto 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1021/acs.chemrev.7b00514 Sukalyan Bhadra 1 , Hisashi Yamamoto 2
Affiliation
|
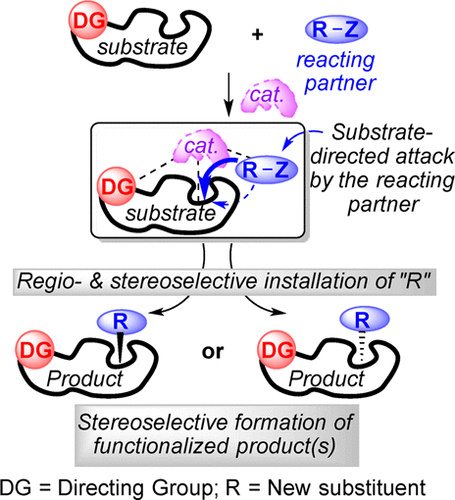
Historically, reagent controlled reactions (mechanism controlled reactions) have played a significant role in the asymmetric synthesis of complex structures. In contrast, today’s asymmetric synthesis is greatly dependent on substrate directed approaches. In this approach, a polar functional group, namely, a “directing group”, in the vicinity of the reactive site inside the substrate has been documented to preassociate with the chiral catalyst, which exerts stereodirecting influence by directing the reacting partner toward one of the enantiotopic faces of the reaction center. Those reactions usually proceed through exceptionally ordered transition states and result in extraordinary levels of stereoselection. Within the last four decades, the substrate directed approach has become an indispensible tool for the preparation of complex chiral frameworks starting directly from relatively simple achiral substrate molecules via asymmetric induction or various resolution techniques or both. Likewise, the substrate directed approach has been applied to functionalize enantiopure substrates bearing pre-exisiting stereocenters into complex structures as a single diastereomer. A classical example is Sharpless asymmetric epoxidation of allylic alcohols in which the free hydroxy function acts as an active anchor to a dimeric Ti-catalyst that controls the stereochemical outcome of the epoxidation process by transferring the oxidant enantioselectively. The principal aim of the present review is to give a general overview of substrate directed asymmetric transformations, a topic that has not yet been documented in the form of a concise review of recently developed approaches. Due to the large number of related applications, only recent advances that have been documented within the last two decades have been reviewed. Furthermore, in the current review, we have mainly highlighted asymmetric reactions that are controlled by abundant and frequently used directing groups such as hydroxy, amide, and sulfonamide groups. In addition, selected examples of a few important substrate-directed chemo-, regio-, and diastereoselective reactions have also been included in this review.
中文翻译:

底物定向不对称反应
从历史上看,试剂控制的反应(机理控制的反应)在复杂结构的不对称合成中发挥了重要作用。相反,当今的不对称合成很大程度上取决于底物定向方法。在这种方法中,已证明在底物内部反应位点附近的极性官能团(即“导向基团”)与手性催化剂预缔合,该手性催化剂通过将反应伴侣导向其中一个分子而产生立体定向影响。反应中心的对映体面。这些反应通常会通过异常有序的过渡态进行,并导致异常的立体选择水平。在过去的40年中,底物定向方法已成为通过不对称诱导或各种拆分技术或两者直接从相对简单的非手性底物分子开始制备复杂手性骨架的必不可少的工具。同样,已采用底物定向方法将带有预先存在的立体中心的对映纯底物功能化为单一非对映异构体的复杂结构。一个典型的例子是烯丙基醇的Sharpless不对称环氧化,其中游离羟基官能团充当二聚Ti催化剂的活性锚,该催化剂通过对映选择性地转移氧化剂来控制环氧化过程的立体化学结果。本综述的主要目的是对底物定向的不对称转化进行总体概述,尚未以简要回顾最近开发的方法的形式记录的主题。由于相关申请的数量众多,因此仅回顾了过去二十年中记录的最新进展。此外,在当前的综述中,我们主要强调了不对称反应,该反应受大量且经常使用的指导基团(例如羟基,酰胺基和磺酰胺基)控制。此外,本综述还包括一些重要的底物导向的化学,区域和非对映选择性反应的实例。在当前的综述中,我们主要强调了不对称反应,该反应受大量且经常使用的指导基团(例如羟基,酰胺基和磺酰胺基)控制。此外,本综述还包括一些重要的底物导向的化学,区域和非对映选择性反应的实例。在当前的综述中,我们主要强调了不对称反应,该反应受大量且经常使用的指导基团(例如羟基,酰胺基和磺酰胺基)控制。此外,本综述还包括一些重要的底物导向的化学,区域和非对映选择性反应的实例。
更新日期:2018-03-23
中文翻译:

底物定向不对称反应
从历史上看,试剂控制的反应(机理控制的反应)在复杂结构的不对称合成中发挥了重要作用。相反,当今的不对称合成很大程度上取决于底物定向方法。在这种方法中,已证明在底物内部反应位点附近的极性官能团(即“导向基团”)与手性催化剂预缔合,该手性催化剂通过将反应伴侣导向其中一个分子而产生立体定向影响。反应中心的对映体面。这些反应通常会通过异常有序的过渡态进行,并导致异常的立体选择水平。在过去的40年中,底物定向方法已成为通过不对称诱导或各种拆分技术或两者直接从相对简单的非手性底物分子开始制备复杂手性骨架的必不可少的工具。同样,已采用底物定向方法将带有预先存在的立体中心的对映纯底物功能化为单一非对映异构体的复杂结构。一个典型的例子是烯丙基醇的Sharpless不对称环氧化,其中游离羟基官能团充当二聚Ti催化剂的活性锚,该催化剂通过对映选择性地转移氧化剂来控制环氧化过程的立体化学结果。本综述的主要目的是对底物定向的不对称转化进行总体概述,尚未以简要回顾最近开发的方法的形式记录的主题。由于相关申请的数量众多,因此仅回顾了过去二十年中记录的最新进展。此外,在当前的综述中,我们主要强调了不对称反应,该反应受大量且经常使用的指导基团(例如羟基,酰胺基和磺酰胺基)控制。此外,本综述还包括一些重要的底物导向的化学,区域和非对映选择性反应的实例。在当前的综述中,我们主要强调了不对称反应,该反应受大量且经常使用的指导基团(例如羟基,酰胺基和磺酰胺基)控制。此外,本综述还包括一些重要的底物导向的化学,区域和非对映选择性反应的实例。在当前的综述中,我们主要强调了不对称反应,该反应受大量且经常使用的指导基团(例如羟基,酰胺基和磺酰胺基)控制。此外,本综述还包括一些重要的底物导向的化学,区域和非对映选择性反应的实例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号