当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heteropoly acid functionalized fluoroelastomer with outstanding chemical durability and performance for vehicular fuel cells†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1039/c8ee00545a Andrew R. Motz 1, 2, 3, 4 , Mei-Chen Kuo 1, 2, 3, 4 , James L. Horan 1, 2, 3, 4 , Rameshwar Yadav 4, 5, 6 , Soenke Seifert 4, 7, 8, 9, 10 , Tara P. Pandey 1, 2, 3, 4 , Samuel Galioto 1, 2, 3, 4 , Yuan Yang 2, 3, 4, 11 , Nilesh V. Dale 4, 5, 6 , Steven J. Hamrock 4, 12, 13 , Andrew M. Herring 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1039/c8ee00545a Andrew R. Motz 1, 2, 3, 4 , Mei-Chen Kuo 1, 2, 3, 4 , James L. Horan 1, 2, 3, 4 , Rameshwar Yadav 4, 5, 6 , Soenke Seifert 4, 7, 8, 9, 10 , Tara P. Pandey 1, 2, 3, 4 , Samuel Galioto 1, 2, 3, 4 , Yuan Yang 2, 3, 4, 11 , Nilesh V. Dale 4, 5, 6 , Steven J. Hamrock 4, 12, 13 , Andrew M. Herring 1, 2, 3, 4
Affiliation
|
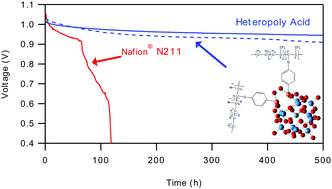
To further facilitate commercialization of automotive fuel cells, durability concerns need to be addressed. Currently the addition of a mechanical support in the membrane is able to adequately solve issues of mechanical degradation, but chemical degradation via oxygenated radical attack remains an unsolved challenge. Typical mitigation strategies use cerium or manganese species to serve as radical scavengers, but these ions are able to migrate in the membrane and even leach out of the system. The approach used in this study is to covalently link and immobilize a heteropoly acid (HPA), more specifically 11-silicotungstic acid (HSiW11), a lacunary HPA of the Keggin structure to a fluoroelastomer, serving as both a radical decomposition catalyst and the proton conducting acid. This dual functionality allows for both high content of radical scavenging species and high ion-exchange capacity. An efficient three step, high yield (77%), commercially viable synthesis for this polymer is reported. The synthesis route for making this new heteropoly acid functionalized polymer is confirmed using infrared spectroscopy (IR), nuclear magnetic resonance (NMR) spectroscopy, and thermogravimetric analysis (TGA). The material exhibits clustering of the HSiW11 moieties, resulting in a poorly connected proton conducting phase when dry, but excellent conductivity is achieved at elevated humidities (0.298 S cm−1 at 80 °C and 95% RH). The proton conductivity shows an enhancement above 60 °C due to a softening of the polymer, as shown by DSC. Under an aggressive chemical accelerated stress test (AST), 90 °C, 30% RH, zero current, and pure O2, the PolyHPA losses only 0.05 V of open circuit voltage (OCV) after 500 h, greatly out performing any other material reported in the literature. For comparison, the Nafion® N211 fuel cell drops below 0.8 V after only 76 h under the same conditions. In fuel cell testing the PolyHPAs have outstanding chemical stability and also possess very low in situ high frequency resistance (HFR) leading to high performance (1.14 W cm−2 at 2 A cm−2), compared to 1.11 W cm−2 for the Nafion® N211 fuel cell at the same current. At 75 wt% HSiW11 loading, the fuel cell HFR showed a 22% decrease over N211.
中文翻译:

杂多酸官能化的含氟弹性体,具有出色的化学耐久性和用于车辆燃料电池的性能†
为了进一步促进汽车燃料电池的商业化,需要解决耐久性问题。目前,在膜中添加机械支撑物能够充分解决机械降解问题,但是通过氧化自由基的攻击仍然是一个尚未解决的挑战。典型的缓解策略是使用铈或锰作为自由基清除剂,但是这些离子能够在膜中迁移,甚至从系统中浸出。本研究中使用的方法是共价连接并固定杂多酸(HPA),更具体地讲是11-硅钨酸(HSiW11)(一种具有Keggin结构的缩孔HPA)与氟弹性体,既可作为自由基分解催化剂,又可作为质子导电酸。这种双重功能既可以实现高含量的自由基清除物质,又可以实现高离子交换能力。据报道该聚合物的有效的三步,高收率(77%),商业上可行的合成。使用红外光谱(IR),核磁共振(NMR)光谱和热重分析(TGA)确认了制备这种新型杂多酸官能化聚合物的合成路线。该材料表现出HSiW11部分的聚集,导致干燥时质子传导相连接不良,但在较高的湿度(0.298 S cm-1在80°C和95%RH下)。如DSC所示,由于聚合物的软化,质子电导率显示出高于60℃的增强。在积极的化学加速应力测试(AST),90°C,30%RH,零电流和纯O 2的条件下,PolyHPA在500 h后仅损失0.05 V的开路电压(OCV),大大超过了其他任何材料文献报道。为了进行比较,Nafion®N211燃料电池在相同条件下仅运行76小时后便降至0.8 V以下。在燃料电池测试PolyHPAs具有突出的化学稳定性和也很低具有原位高频电阻(HFR)导致高性能(1.14 w ^厘米-2以2A厘米-2)相比,1.11W¯¯厘米-2用于Nafion®N211燃料电池,电流相同。在HSiW11负载为75 wt%的情况下,燃料电池HFR较N211降低了22%。
更新日期:2018-03-23
中文翻译:

杂多酸官能化的含氟弹性体,具有出色的化学耐久性和用于车辆燃料电池的性能†
为了进一步促进汽车燃料电池的商业化,需要解决耐久性问题。目前,在膜中添加机械支撑物能够充分解决机械降解问题,但是通过氧化自由基的攻击仍然是一个尚未解决的挑战。典型的缓解策略是使用铈或锰作为自由基清除剂,但是这些离子能够在膜中迁移,甚至从系统中浸出。本研究中使用的方法是共价连接并固定杂多酸(HPA),更具体地讲是11-硅钨酸(HSiW11)(一种具有Keggin结构的缩孔HPA)与氟弹性体,既可作为自由基分解催化剂,又可作为质子导电酸。这种双重功能既可以实现高含量的自由基清除物质,又可以实现高离子交换能力。据报道该聚合物的有效的三步,高收率(77%),商业上可行的合成。使用红外光谱(IR),核磁共振(NMR)光谱和热重分析(TGA)确认了制备这种新型杂多酸官能化聚合物的合成路线。该材料表现出HSiW11部分的聚集,导致干燥时质子传导相连接不良,但在较高的湿度(0.298 S cm-1在80°C和95%RH下)。如DSC所示,由于聚合物的软化,质子电导率显示出高于60℃的增强。在积极的化学加速应力测试(AST),90°C,30%RH,零电流和纯O 2的条件下,PolyHPA在500 h后仅损失0.05 V的开路电压(OCV),大大超过了其他任何材料文献报道。为了进行比较,Nafion®N211燃料电池在相同条件下仅运行76小时后便降至0.8 V以下。在燃料电池测试PolyHPAs具有突出的化学稳定性和也很低具有原位高频电阻(HFR)导致高性能(1.14 w ^厘米-2以2A厘米-2)相比,1.11W¯¯厘米-2用于Nafion®N211燃料电池,电流相同。在HSiW11负载为75 wt%的情况下,燃料电池HFR较N211降低了22%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号