当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrolytic CO2 Reduction in a Flow Cell
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1021/acs.accounts.8b00010 David M. Weekes 1 , Danielle A. Salvatore 2 , Angelica Reyes 2 , Aoxue Huang 1 , Curtis P. Berlinguette 1, 2, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-03-23 00:00:00 , DOI: 10.1021/acs.accounts.8b00010 David M. Weekes 1 , Danielle A. Salvatore 2 , Angelica Reyes 2 , Aoxue Huang 1 , Curtis P. Berlinguette 1, 2, 3
Affiliation
|
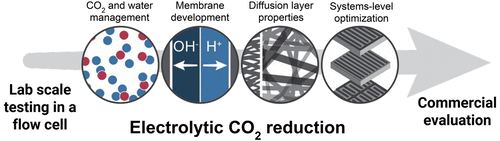
Electrocatalytic CO2 conversion at near ambient temperatures and pressures offers a potential means of converting waste greenhouse gases into fuels or commodity chemicals (e.g., CO, formic acid, methanol, ethylene, alkanes, and alcohols). This process is particularly compelling when driven by excess renewable electricity because the consequent production of solar fuels would lead to a closing of the carbon cycle. However, such a technology is not currently commercially available. While CO2 electrolysis in H-cells is widely used for screening electrocatalysts, these experiments generally do not effectively report on how CO2 electrocatalysts behave in flow reactors that are more relevant to a scalable CO2 electrolyzer system. Flow reactors also offer more control over reagent delivery, which includes enabling the use of a gaseous CO2 feed to the cathode of the cell. This setup provides a platform for generating much higher current densities (J) by reducing the mass transport issues inherent to the H-cells.
中文翻译:

流通池中的电解CO 2还原
在接近环境温度和压力的条件下,电催化的CO 2转化提供了一种潜在的手段,可将温室气体转化为燃料或日用化学品(例如,CO,甲酸,甲醇,乙烯,烷烃和醇)。当由过量的可再生电力驱动时,该过程特别引人注目,因为随后产生的太阳能燃料将导致碳循环的结束。但是,这种技术目前尚不能商业获得。尽管H电池中的CO 2电解被广泛用于筛选电催化剂,但是这些实验通常无法有效地报告CO 2电催化剂在流动反应器中的行为,该反应器与可扩展的CO 2更相关电解系统。流动反应器还提供了对试剂输送的更多控制,这包括使得能够使用气态CO 2进料到电池的阴极。这种设置提供了一个平台,可通过减少H电池固有的传质问题来产生更高的电流密度(J)。
更新日期:2018-03-23
中文翻译:

流通池中的电解CO 2还原
在接近环境温度和压力的条件下,电催化的CO 2转化提供了一种潜在的手段,可将温室气体转化为燃料或日用化学品(例如,CO,甲酸,甲醇,乙烯,烷烃和醇)。当由过量的可再生电力驱动时,该过程特别引人注目,因为随后产生的太阳能燃料将导致碳循环的结束。但是,这种技术目前尚不能商业获得。尽管H电池中的CO 2电解被广泛用于筛选电催化剂,但是这些实验通常无法有效地报告CO 2电催化剂在流动反应器中的行为,该反应器与可扩展的CO 2更相关电解系统。流动反应器还提供了对试剂输送的更多控制,这包括使得能够使用气态CO 2进料到电池的阴极。这种设置提供了一个平台,可通过减少H电池固有的传质问题来产生更高的电流密度(J)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号