当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the nature of organic and inorganic centers that bifurcate electrons, coupling exergonic and endergonic oxidation–reduction reactions
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1039/c8cc01530a John W. Peters 1, 2, 3 , David N. Beratan 3, 4, 5, 5, 6 , Gerrit J. Schut 3, 7, 8 , Michael W. W. Adams 3, 7, 8
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1039/c8cc01530a John W. Peters 1, 2, 3 , David N. Beratan 3, 4, 5, 5, 6 , Gerrit J. Schut 3, 7, 8 , Michael W. W. Adams 3, 7, 8
Affiliation
|
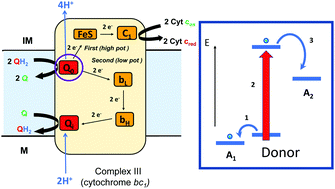
Bifurcating electrons to couple endergonic and exergonic electron-transfer reactions has been shown to have a key role in energy conserving redox enzymes. Bifurcating enzymes require a redox center that is capable of directing electron transport along two spatially separate pathways. Research into the nature of electron bifurcating sites indicates that one of the keys is the formation of a low potential oxidation state to satisfy the energetics required of the endergonic half reaction, indicating that any redox center (organic or inorganic) that can exist in multiple oxidation states with sufficiently separated redox potentials should be capable of electron bifurcation. In this Feature Article, we explore a paradigm for bifurcating electrons down independent high and low potential pathways, and describe redox cofactors that have been demonstrated or implicated in driving this unique biochemistry.
中文翻译:

关于分叉电子的有机中心和无机中心的性质,其能电和负电子的氧化还原反应耦合
分叉的电子与共电子和能电子转移反应已显示出在节能氧化还原酶中的关键作用。分叉酶需要氧化还原中心,该氧化还原中心能够指导电子沿着两个空间上分开的路径传输。对电子分叉位点性质的研究表明,关键之一是形成低电位的氧化态,以满足负电子半反应所需的能量学要求,这表明任何可以存在于多重氧化中的氧化还原中心(有机或无机)具有足够分离的氧化还原电势的态应该能够电子分叉。在本专题文章中,我们探讨了将电子分叉成独立的高和低电势路径的范例,
更新日期:2018-03-22
中文翻译:

关于分叉电子的有机中心和无机中心的性质,其能电和负电子的氧化还原反应耦合
分叉的电子与共电子和能电子转移反应已显示出在节能氧化还原酶中的关键作用。分叉酶需要氧化还原中心,该氧化还原中心能够指导电子沿着两个空间上分开的路径传输。对电子分叉位点性质的研究表明,关键之一是形成低电位的氧化态,以满足负电子半反应所需的能量学要求,这表明任何可以存在于多重氧化中的氧化还原中心(有机或无机)具有足够分离的氧化还原电势的态应该能够电子分叉。在本专题文章中,我们探讨了将电子分叉成独立的高和低电势路径的范例,











































 京公网安备 11010802027423号
京公网安备 11010802027423号