Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-03-22 , DOI: 10.1016/j.bmc.2018.03.032 Dibyendu Mondal , Eric M. Koehn , Jiajun Yao , David F. Wiemer , Amnon Kohen
|
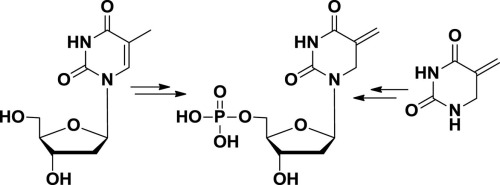
Exocyclic olefin variants of thymidylate (dTMP) recently have been proposed as reaction intermediates for the thymidyl biosynthesis enzymes found in many pathogenic organisms, yet synthetic reports on these materials are lacking. Here we report two strategies to prepare the exocyclic olefin isomer of dTMP, which is a putative reaction intermediate in pathogenic thymidylate biosynthesis and a novel nucleotide analog. Our most effective strategy involves preserving the existing glyosidic bond of thymidine and manipulating the base to generate the exocyclic methylene moiety. We also report a successful enzymatic deoxyribosylation of a non-aromatic nucleobase isomer of thymine, which provides an additional strategy to access nucleotide analogs with disrupted ring conjugation or with reduced heterocyclic bases. The strategies reported here are straightforward and extendable towards the synthesis of various pyrimidine nucleotide analogs, which could lead to compounds of value in studies of enzyme reaction mechanisms or serve as templates for rational drug design.
中文翻译:

化学酶法合成胸苷一磷酸的环外烯烃异构体
最近有人提出了胸苷酸(dTMP)的环外烯烃变体作为许多病原生物中发现的胸苷基生物合成酶的反应中间体,但缺乏有关这些材料的合成报告。在这里,我们报告两种策略来制备dTMP的环外烯烃异构体,它是致病性胸苷酸生物合成中的一种假定的反应中间体和一种新型的核苷酸类似物。我们最有效的策略涉及保留胸腺嘧啶存在的糖苷酸键,并操纵碱基以生成环外亚甲基部分。我们还报告了胸腺嘧啶的非芳香族核碱基异构体的成功的酶促脱氧核糖基化,这提供了一种额外的策略来访问具有破坏的环偶联或减少的杂环碱基的核苷酸类似物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号