当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel strategy for the preparation of 3-perfluoroalkylated-2H-indazole derivatives
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-22 , DOI: 10.1016/j.tetlet.2018.03.051 Hassen Bel Abed , Nils Weißing , Jens Schoene , Jannik Paulus , Norbert Sewald , Marc Nazaré
中文翻译:

制备3-全氟烷基化2 H-吲唑衍生物的新策略
更新日期:2018-03-22
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-03-22 , DOI: 10.1016/j.tetlet.2018.03.051 Hassen Bel Abed , Nils Weißing , Jens Schoene , Jannik Paulus , Norbert Sewald , Marc Nazaré
|
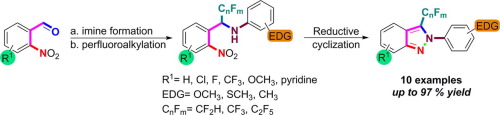
A simple and novel methodology for the synthesis of 3-perfluoroalkylated-2H-indazole derivatives has been elaborated. The perfluoroalkylation of readily available 2-nitrobenzaldimines bearing electron donating groups was performed using the Ruppert-Prakash reagent and its analogues to afford α-difluoromethylated, α-trifluoromethylated and α-pentafluoroethylated benzylamines. A final reductive cyclization mediated by SnCl2·2H2O led to 2H-indazoles containing perfluoroalkyl groups via the generation of a new NN bond in moderate to good yields.
中文翻译:

制备3-全氟烷基化2 H-吲唑衍生物的新策略
已经阐述了合成3-全氟烷基化的2- H-吲唑衍生物的简单新颖的方法。使用Ruppert-Prakash试剂及其类似物对带有供电子基团的2-硝基苯甲二胺进行全氟烷基化,得到α-二氟甲基化,α-三氟甲基化和α-五氟乙基化的苄胺。由SnCl 2 ·2H 2 O介导的最终还原性环化反应通过生成新的N N键,以中等至良好的收率生成了2个含H-吲唑的全氟烷基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号