当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of Criegee Intermediate with Nitric Acid at the Air-Water Interface
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-22 , DOI: 10.1021/jacs.8b01191 Manoj Kumar 1 , Jie Zhong 1 , Xiao Cheng Zeng 1, 2, 3 , Joseph S. Francisco 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-22 , DOI: 10.1021/jacs.8b01191 Manoj Kumar 1 , Jie Zhong 1 , Xiao Cheng Zeng 1, 2, 3 , Joseph S. Francisco 1
Affiliation
|
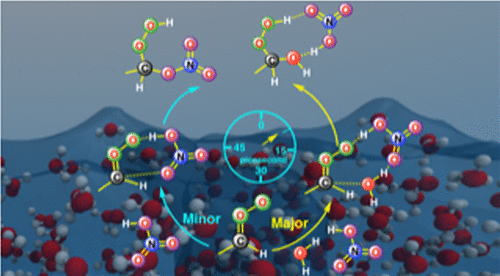
The role of aqueous surfaces in promoting atmospheric chemistry is increasingly being recognized. However, the bimolecular chemistries of Criegee intermediates, which influence the tropospheric budget of OH radicals, organic acids, hydroperoxides, nitrates, sulfates, and particulate material, remain less explored on an aqueous surface. Herein we have employed Born-Oppenheimer molecular dynamics simulations and two-layer ONIOM (QM:MM) in an electronic embedding scheme to study the reaction and the spectroscopic signal of anti-CH3CHOO with nitric acid (HNO3) at the air-water interface, which is expected to be an important reaction in polluted urban environments. The results reveal that on the water surface, the HNO3-mediated hydration of anti-CH3CHOO is the most dominant pathway, whereas the traditionally believed direct reaction between anti-CH3CHOO and HNO3, which results in the formation of nitrooxyethyl hydroperoxide, is only the minor channel. Both reaction pathways follow a stepwise mechanism at the air-water interface and occur on the picosecond time scale. These new reactions are expected to be relevant in the hazy environments of globally polluted urban regions where nitrates and sulfates are abundantly present. During the hazy period, the high relative humidity and the presence of fog droplets may favor the HNO3-mediated Criegee hydration over the nitrooxyethyl hydroperoxide forming reaction. A similar reaction mechanism with Criegee intermediates could be expected on the water surface for organic acids, which possess HNO3-like functionalities, and may play a role in improving our knowledge of the organic acid budget in the terrestrial equatorial regions and high northern latitudes. The ONIOM calculations suggest that the N-O stretching bands around 1600-1200 cm-1 and NO2 bending band around 750 cm-1 in nitrooxyethyl hydroperoxide could be used as spectroscopic markers for distinguishing it from hydrooxyethyl hydroperoxide on the water surface.
中文翻译:

Criegee 中间体与硝酸在气水界面反应
水表面在促进大气化学方面的作用越来越得到认可。然而,影响 OH 自由基、有机酸、氢过氧化物、硝酸盐、硫酸盐和颗粒物质的对流层预算的 Criegee 中间体的双分子化学在水表面上的研究较少。在此,我们在电子嵌入方案中采用 Born-Oppenheimer 分子动力学模拟和两层 ONIOM (QM:MM) 来研究反 CH3CHOO 与硝酸 (HNO3) 在空气-水界面的反应和光谱信号,预计这将是受污染的城市环境中的重要反应。结果表明,在水面,HNO3介导的anti-CH3CHOO水合作用是最主要的途径,而传统上认为抗 CH3CHOO 和 HNO3 之间的直接反应会导致硝基氧乙基氢过氧化物的形成,这只是次要通道。两种反应途径都遵循空气-水界面处的逐步机制,并在皮秒时间尺度上发生。预计这些新反应将与硝酸盐和硫酸盐大量存在的全球污染城市地区的朦胧环境相关。在雾霾期间,较高的相对湿度和雾滴的存在可能有利于 HNO3 介导的 Criegee 水合反应而不是硝基氧乙基氢过氧化物形成反应。对于具有类似 HNO3 的功能的有机酸,可以预期在水表面上与 Criegee 中间体类似的反应机制,并且可能有助于提高我们对陆地赤道地区和北高纬度地区有机酸收支的了解。ONIOM 计算表明,硝基氧乙基氢过氧化物中 1600-1200 cm-1 附近的 NO 伸缩带和 750 cm-1 附近的 NO2 弯曲带可用作光谱标记,以将其与水表面的氢氧乙基氢过氧化物区分开来。
更新日期:2018-03-22
中文翻译:

Criegee 中间体与硝酸在气水界面反应
水表面在促进大气化学方面的作用越来越得到认可。然而,影响 OH 自由基、有机酸、氢过氧化物、硝酸盐、硫酸盐和颗粒物质的对流层预算的 Criegee 中间体的双分子化学在水表面上的研究较少。在此,我们在电子嵌入方案中采用 Born-Oppenheimer 分子动力学模拟和两层 ONIOM (QM:MM) 来研究反 CH3CHOO 与硝酸 (HNO3) 在空气-水界面的反应和光谱信号,预计这将是受污染的城市环境中的重要反应。结果表明,在水面,HNO3介导的anti-CH3CHOO水合作用是最主要的途径,而传统上认为抗 CH3CHOO 和 HNO3 之间的直接反应会导致硝基氧乙基氢过氧化物的形成,这只是次要通道。两种反应途径都遵循空气-水界面处的逐步机制,并在皮秒时间尺度上发生。预计这些新反应将与硝酸盐和硫酸盐大量存在的全球污染城市地区的朦胧环境相关。在雾霾期间,较高的相对湿度和雾滴的存在可能有利于 HNO3 介导的 Criegee 水合反应而不是硝基氧乙基氢过氧化物形成反应。对于具有类似 HNO3 的功能的有机酸,可以预期在水表面上与 Criegee 中间体类似的反应机制,并且可能有助于提高我们对陆地赤道地区和北高纬度地区有机酸收支的了解。ONIOM 计算表明,硝基氧乙基氢过氧化物中 1600-1200 cm-1 附近的 NO 伸缩带和 750 cm-1 附近的 NO2 弯曲带可用作光谱标记,以将其与水表面的氢氧乙基氢过氧化物区分开来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号