Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2018-03-22 , DOI: 10.1016/j.cej.2018.03.114 Jie Ding , Siqun Tang , Xiao Chen , Mu Ding , Jinyang Kang , Rulei Wu , Zhihai Fu , Yongdong Jin , Laicai Li , Xiaojie Feng , Ruibing Wang , Chuanqin Xia
|
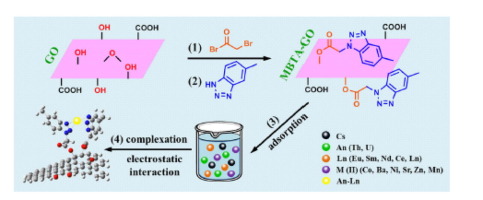
The removal of actinide (An) and lanthanide (Ln) elements from aqueous solution is a significant strategy for the safe disposal of radioactive wastes. Herein we presented a 5-methylbenzotriazole modified graphene oxide (MBTA-GO) as a novel adsorbent, in which benzotriazole group was used as targeted sorption site bearing strong affinity toward An-Ln (An and Ln) ions for the first time. MBTA-GO was characterized by Fourier transformed infrared spectroscopy (FT-IR), thermal gravimetric analysis (TGA) and X-ray photoelectron spectroscopy (XPS). The effect of pH and initial concentration on combined adsorption of An-Ln were examined, and the results showed that the adsorption of metal ions on MBTA-GO was strongly dependent on pH in multi-cation solution, and MBTA-GO exhibited excellent An-Ln selectivity (selectivity coefficients are more than 99%), overwhelming majority of other reported adsorbents. The cycle experiments revealed that the MBTA-GO exhibited good reusability and structural stability. By density functional theory (DFT) calculation, the binding energy of Cation-MBTA-GO followed the order of Th(IV) > U(VI) > Eu(III) > La(III) > Sr(II) > Cs(I), suggesting that the benzotriazole groups were preferentially responsible for An-Ln ions on MBTA-GO, which is in well agreement with the experimental results. Finally, the mechanism of selective coadsorption was revealed, which is the synergistic effect of chemical complexation and electrostatic interaction.
中文翻译:

将苯并三唑引入氧化石墨烯中以实现An和Ln的高选择性共吸附:易于合成和理论研究
从水溶液中去除act系元素(An)和镧系元素(Ln)是安全处置放射性废物的重要策略。本文中,我们提出了一种5-甲基苯并三唑改性的氧化石墨烯(MBTA-GO)作为新型吸附剂,其中苯并三唑基团首次用作对An-Ln(An和Ln)离子具有强亲和力的目标吸附位点。MBTA-GO的特征在于傅立叶变换红外光谱(FT-IR),热重分析(TGA)和X射线光电子能谱(XPS)。研究了pH和初始浓度对An-Ln联合吸附的影响,结果表明MBTA-GO上金属离子的吸附强烈依赖于多阳离子溶液中的pH,MBTA-GO表现出优异的An-Ln Ln选择性(选择性系数大于99%),绝大多数其他报告的吸附剂。循环实验表明,MBTA-GO具有良好的可重用性和结构稳定性。通过密度泛函理论(DFT)计算,阳离子-MBTA-GO的结合能遵循Th(IV)> U(VI)> Eu(III)> La(III)> Sr(II)> Cs(I ),表明苯并三唑基团优先负责MBTA-GO上的An-Ln离子,这与实验结果非常吻合。最后,揭示了选择性共吸附的机理,这是化学络合和静电相互作用的协同作用。Cation-MBTA-GO的结合能遵循Th(IV)> U(VI)> Eu(III)> La(III)> Sr(II)> Cs(I)的顺序,表明苯并三唑基团优先负责MBTA-GO上的An-Ln离子,这与实验结果非常吻合。最后,揭示了选择性共吸附的机理,这是化学络合和静电相互作用的协同作用。Cation-MBTA-GO的结合能遵循Th(IV)> U(VI)> Eu(III)> La(III)> Sr(II)> Cs(I)的顺序,表明苯并三唑基团优先负责MBTA-GO上的An-Ln离子,这与实验结果非常吻合。最后,揭示了选择性共吸附的机理,这是化学络合和静电相互作用的协同作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号