当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A pilot study in humans of microneedle sensor arrays for continuous glucose monitoring
Analytical Methods ( IF 2.7 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8ay00264a Sanjiv Sharma 1, 2, 3 , Ahmed El-Laboudi 2, 4, 5 , Monika Reddy 2, 4, 5 , Narvada Jugnee 2, 4, 5 , Sujan Sivasubramaniyam 2, 4, 5 , Mohamed El Sharkawy 2, 6, 7 , Pantelis Georgiou 2, 6, 7 , Desmond Johnston 2, 4, 5 , Nick Oliver 2, 4, 5 , Anthony E. G. Cass 1, 2, 3
Analytical Methods ( IF 2.7 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8ay00264a Sanjiv Sharma 1, 2, 3 , Ahmed El-Laboudi 2, 4, 5 , Monika Reddy 2, 4, 5 , Narvada Jugnee 2, 4, 5 , Sujan Sivasubramaniyam 2, 4, 5 , Mohamed El Sharkawy 2, 6, 7 , Pantelis Georgiou 2, 6, 7 , Desmond Johnston 2, 4, 5 , Nick Oliver 2, 4, 5 , Anthony E. G. Cass 1, 2, 3
Affiliation
|
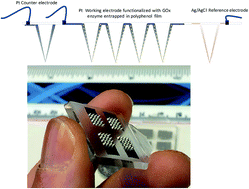
Although subcutaneously implanted continuous glucose monitoring (CGM) devices have been shown to support diabetes self-management, their uptake remains low due to a combination of high manufacturing cost and limited accuracy and precision arising from their invasiveness. To address these points, minimally invasive, a solid microneedle array-based sensor for continuous glucose monitoring is reported here. These intradermal solid microneedle CGM sensors are designed for low cost manufacturing. The tolerability and performance of these devices is demonstrated through clinical studies, both in healthy volunteers and participants with type 1 diabetes (T1D). The geometry of these solid microneedles allows them to penetrate dermal tissue without the need for an applicator. The outer surface of these solid microneedles are modified as glucose biosensors. The microneedles sit in the interstitial fluid of the skin compartment and monitor real-time changes in glucose concentration. Optical coherence tomography measurements revealed no major axial movement of the microneedles in the tissue. No significant adverse events were observed and low pain scores were reported when compared to catheter insertion, deeming it safe for clinical studies in T1D. These amperometric sensors also yielded currents that tracked venous blood glucose concentrations, showing a clinically acceptable correlation. Studies in people with T1D gave a mean absolute relative difference (MARD) of 9% (with respect to venous blood glucose) with over 94% of the data points in the A and B zones of the Clarke error grid. These findings provide baseline data for further device development and a larger clinical efficacy and acceptability study of this microneedle intradermal glucose sensor in T1D.
中文翻译:

微针传感器阵列在人体中进行连续葡萄糖监测的初步研究
尽管已证明皮下植入的连续葡萄糖监测(CGM)设备可支持糖尿病的自我管理,但由于其制造成本高,侵入性有限而导致精度和精度受限,因此它们的摄取率仍然很低。为了解决这些问题,这里报道了一种微创的,基于固体微针阵列的传感器,用于连续葡萄糖监测。这些皮内固体微针CGM传感器专为低成本制造而设计。在健康志愿者和1型糖尿病(T1D)参与者中,通过临床研究证明了这些设备的耐受性和性能。这些固体微针的几何形状使它们可以穿透皮肤组织,而无需施加器。这些固体微针的外表面被修饰为葡萄糖生物传感器。微针位于皮肤隔室的组织液中,并监测葡萄糖浓度的实时变化。光学相干断层扫描测量显示组织中的微针没有大的轴向运动。与导管插入相比,未观察到明显的不良事件,并且疼痛评分较低,被认为可用于T1D的临床研究。这些安培传感器还产生跟踪静脉血糖浓度的电流,显示出临床上可接受的相关性。T1D患者的研究得出的平均绝对相对差异(MARD)为9%(相对于静脉血糖),其中Clarke误差网格的A区和B区的数据点超过94%。
更新日期:2018-03-21
中文翻译:

微针传感器阵列在人体中进行连续葡萄糖监测的初步研究
尽管已证明皮下植入的连续葡萄糖监测(CGM)设备可支持糖尿病的自我管理,但由于其制造成本高,侵入性有限而导致精度和精度受限,因此它们的摄取率仍然很低。为了解决这些问题,这里报道了一种微创的,基于固体微针阵列的传感器,用于连续葡萄糖监测。这些皮内固体微针CGM传感器专为低成本制造而设计。在健康志愿者和1型糖尿病(T1D)参与者中,通过临床研究证明了这些设备的耐受性和性能。这些固体微针的几何形状使它们可以穿透皮肤组织,而无需施加器。这些固体微针的外表面被修饰为葡萄糖生物传感器。微针位于皮肤隔室的组织液中,并监测葡萄糖浓度的实时变化。光学相干断层扫描测量显示组织中的微针没有大的轴向运动。与导管插入相比,未观察到明显的不良事件,并且疼痛评分较低,被认为可用于T1D的临床研究。这些安培传感器还产生跟踪静脉血糖浓度的电流,显示出临床上可接受的相关性。T1D患者的研究得出的平均绝对相对差异(MARD)为9%(相对于静脉血糖),其中Clarke误差网格的A区和B区的数据点超过94%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号