当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient and reversible absorption of ammonia by cobalt ionic liquids through Lewis acid–base and cooperative hydrogen bond interactions
Green Chemistry ( IF 9.3 ) Pub Date : 2018-03-22 , DOI: 10.1039/c8gc00215k Shaojuan Zeng 1, 2, 3, 4, 5 , Lei Liu 6, 7, 8, 9, 10 , Dawei Shang 1, 2, 3, 4, 5 , Jianpeng Feng 1, 2, 3, 4, 5 , Haifeng Dong 1, 2, 3, 4, 5 , Qiuxia Xu 1, 2, 3, 4, 5 , Xiangping Zhang 1, 2, 3, 4, 5 , Suojiang Zhang 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2018-03-22 , DOI: 10.1039/c8gc00215k Shaojuan Zeng 1, 2, 3, 4, 5 , Lei Liu 6, 7, 8, 9, 10 , Dawei Shang 1, 2, 3, 4, 5 , Jianpeng Feng 1, 2, 3, 4, 5 , Haifeng Dong 1, 2, 3, 4, 5 , Qiuxia Xu 1, 2, 3, 4, 5 , Xiangping Zhang 1, 2, 3, 4, 5 , Suojiang Zhang 1, 2, 3, 4, 5
Affiliation
|
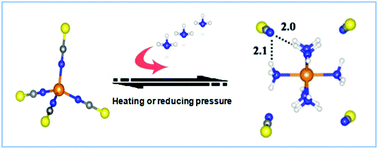
Ammonia (NH3) emissions have caused a wide range of environmental problems and serious harm to human health. However, efficiently separating NH3 and simultaneously recovering high purity NH3 easily remains a great challenge. A new strategy to design transition metal ionic liquids (MILs) by combining specific metal centers and ligands with ILs was proposed for efficient and reversible absorption of NH3. Not only exceptional NH3 absorption capacity and high NH3/CO2 selectivity, but also excellent recyclability were achieved by cobalt ILs [Cnmim]2[Co(NCS)4]. The maximal capacity of NH3 is up to 6.09 mol NH3 mol IL−1 at 30 °C and 0.10 MPa, which is much higher than all reported ILs to date, and is over 30 times higher than the conventional ILs [Cnmim][SCN]. The superior NH3 capacity and desorption performance originate from the moderate Lewis acid–base and cooperative hydrogen bond interactions between the metal center-ligands and NH3.
中文翻译:

通过路易斯酸碱和协同氢键相互作用,钴离子液体有效且可逆地吸收氨
氨(NH 3)的排放已引起广泛的环境问题,并严重危害人类健康。然而,有效地分离NH 3并同时回收高纯度NH 3仍然是巨大的挑战。提出了一种通过结合特定的金属中心和配体与IL来设计过渡金属离子液体(MIL)的新策略,以有效且可逆地吸收NH 3。不仅特殊NH 3的吸收容量和高NH 3 / CO 2的选择性,而且具有优异的再利用性是由钴离子液体[C实现Ñ MIM] 2 [CO(NCS)4]。在30°C和0.10 MPa下,NH 3的最大容量高达6.09 mol NH 3 mol IL -1,这比迄今报道的所有IL都高得多,并且比常规IL高30倍以上[C n mim ] [SCN]。优越的NH 3容量和解吸性能源于金属中心配体与NH 3之间适度的路易斯酸碱和协同氢键相互作用。
更新日期:2018-05-09
中文翻译:

通过路易斯酸碱和协同氢键相互作用,钴离子液体有效且可逆地吸收氨
氨(NH 3)的排放已引起广泛的环境问题,并严重危害人类健康。然而,有效地分离NH 3并同时回收高纯度NH 3仍然是巨大的挑战。提出了一种通过结合特定的金属中心和配体与IL来设计过渡金属离子液体(MIL)的新策略,以有效且可逆地吸收NH 3。不仅特殊NH 3的吸收容量和高NH 3 / CO 2的选择性,而且具有优异的再利用性是由钴离子液体[C实现Ñ MIM] 2 [CO(NCS)4]。在30°C和0.10 MPa下,NH 3的最大容量高达6.09 mol NH 3 mol IL -1,这比迄今报道的所有IL都高得多,并且比常规IL高30倍以上[C n mim ] [SCN]。优越的NH 3容量和解吸性能源于金属中心配体与NH 3之间适度的路易斯酸碱和协同氢键相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号