当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Al-distribution on oxygen activation over Cu–CHA†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1039/c8cy00083b Lin Chen 1, 2, 3, 4 , Hanne Falsig 5, 6, 7 , Ton V. W. Janssens 5, 6, 7 , Jonas Jansson 4, 8, 9 , Magnus Skoglundh 2, 3, 4, 10, 11 , Henrik Grönbeck 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1039/c8cy00083b Lin Chen 1, 2, 3, 4 , Hanne Falsig 5, 6, 7 , Ton V. W. Janssens 5, 6, 7 , Jonas Jansson 4, 8, 9 , Magnus Skoglundh 2, 3, 4, 10, 11 , Henrik Grönbeck 1, 2, 3, 4
Affiliation
|
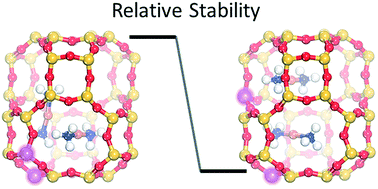
Cu(NH3)2+-pairs in chabazite (CHA) have been suggested to activate oxygen during low-temperature selective catalytic reduction of nitrogen oxides with ammonia (NH3-SCR). As charge neutrality requires that each Cu-complex is associated with a framework Al, the Al-distribution may affect Cu(NH3)2+-pair formation and subsequent oxygen activation. Here, density functional theory calculations in combination with ab initio molecular dynamics simulations are used to explore Cu(NH3)2+-pair formation and oxygen activation in Cu–CHA. The Al-distribution is found to markedly affect the probability for Cu(NH3)2+-pair formation. Moreover, the molecular dynamics simulations reveal a low-energy reaction path for O2 activation and dissociation. The facile O2 dissociation suggests that Cu-pair formation rather than O2 activation governs the low-temperature NH3-SCR activity. The results indicate that precise synthesis of Cu-exchanged chabazite with respect to Al-distribution may enhance the catalytic activity.
中文翻译:

Al分布对Cu–CHA上氧活化的影响†
已经提出菱沸石(CHA)中的Cu(NH 3)2 +-对在用氨(NH 3 -SCR)低温选择性催化还原氮氧化物的过程中活化氧。由于电荷中和要求每个Cu-络合物都与骨架Al缔合,因此Al的分布可能会影响Cu(NH 3)2 +对的形成以及随后的氧活化。在这里,密度泛函理论计算与从头算分子动力学模拟相结合,用于研究Cu(NH 3)2 +Cu-CHA中的双键对形成和氧活化。发现Al分布明显影响Cu(NH 3)2 +-对形成的可能性。此外,分子动力学模拟揭示了O 2活化和离解的低能反应路径。容易的O 2解离表明,Cu对的形成而不是O 2的活化控制着低温NH 3 -SCR的活性。结果表明,就Al分布而言,精确合成Cu交换菱沸石可以增强催化活性。
更新日期:2018-03-22
中文翻译:

Al分布对Cu–CHA上氧活化的影响†
已经提出菱沸石(CHA)中的Cu(NH 3)2 +-对在用氨(NH 3 -SCR)低温选择性催化还原氮氧化物的过程中活化氧。由于电荷中和要求每个Cu-络合物都与骨架Al缔合,因此Al的分布可能会影响Cu(NH 3)2 +对的形成以及随后的氧活化。在这里,密度泛函理论计算与从头算分子动力学模拟相结合,用于研究Cu(NH 3)2 +Cu-CHA中的双键对形成和氧活化。发现Al分布明显影响Cu(NH 3)2 +-对形成的可能性。此外,分子动力学模拟揭示了O 2活化和离解的低能反应路径。容易的O 2解离表明,Cu对的形成而不是O 2的活化控制着低温NH 3 -SCR的活性。结果表明,就Al分布而言,精确合成Cu交换菱沸石可以增强催化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号