Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-03-20 , DOI: 10.1016/j.mcat.2018.02.017 Claudia Sanfilippo , Alfio Adriano Paternò , Angela Patti
|
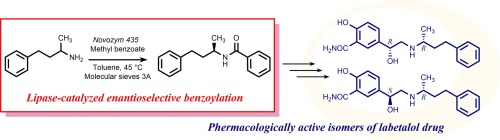
Lipase-catalyzed benzoylation of amines was shown to be feasible, in some cases with high enantioselectivity, and the best results were obtained using immobilized lipase from Candida antarctica (Novozym 435) and methyl benzoate as acyl donor in the presence of molecular sieves. The procedure was optimized for the resolution of (±)-1-methyl-3-phenylpropylamine, a key intermediate in the synthesis of antihypertensive drug labetalol, and the enantiopure (R)-benzamide was then converted into the pharmacologically active isomers of the drug. In comparison with the reported synthesis of chiral isomers of labetalol, this chemoenzymatic route offers the advantage in the lack of any chiral stoichiometric auxiliary.
中文翻译:

通过脂肪酶催化的苯甲酰化拆分外消旋胺:拉贝洛尔药理活性异构体的化学酶法合成
脂肪酶催化的胺的苯甲酰化是可行的,在某些情况下具有高对映选择性,在分子筛存在下,使用南极假丝酵母的固定化脂肪酶(Novozym 435)和苯甲酸甲酯作为酰基供体,可获得最佳结果。优化了程序,以分离(±)-1-甲基-3-苯基丙胺,这是合成降压药拉贝洛尔的关键中间体,然后将对映纯(R)-苯甲酰胺转化为该药物的药理活性异构体。与已报道的拉贝洛尔手性异构体的合成相比,该化学酶途径的优势在于没有任何手性化学计量助剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号