Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-03-21 , DOI: 10.1016/j.bioorg.2018.03.015 Guangcheng Wang , Zhiyun Peng , Zipeng Gong , Yongjun Li
|
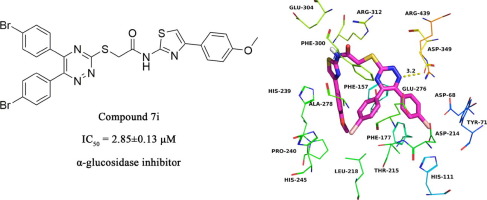
A novel 5,6-diaryl-1,2,4-triazine thiazole derivatives (7a-7q) were synthesized and characterized by 1H NMR and 13C NMR and evaluated for their α-glucosidase inhibitory activity. All tested compounds displayed good α-glucosidase inhibitory activity with IC50 values ranging between 2.85 ± 0.13 and 14.19 ± 0.23 μM when compared to the standard drug acarbose (IC50 = 817.38 ± 6.27 μM). Compound 7i (IC50 = 2.85 ± 0.13 μM) exhibited the highest activity among this series of compounds. Molecular docking studies were carried out in order to investigate the binding mode of this class of compounds to α-glucosidase. This study showed that these 5,6-diaryl-1,2,4-triazine thiazole derivatives are a new class of α-glucosidase inhibitors.
中文翻译:

新型5,6-二芳基-1,2,4-三嗪噻唑衍生物作为新型α-葡萄糖苷酶抑制剂的合成,生物学评估和对接研究
合成了新颖的5,6-二芳基-1,2,4-三嗪噻唑衍生物(7a - 7q),并通过1 H NMR和13 C NMR对其进行了表征,并评估了其对α-葡萄糖苷酶的抑制活性。与标准阿卡波糖(IC 50 = 817.38±6.27μM)相比,所有测试化合物均显示出良好的α-葡萄糖苷酶抑制活性,IC 50值在2.85±0.13和14.19± 0.23μM之间。化合物7i(IC 50 = 2.85±0.13μM)在这一系列化合物中表现出最高的活性。为了研究这类化合物与α-葡萄糖苷酶的结合方式,进行了分子对接研究。该研究表明,这些5,6-二芳基-1,2,4-三嗪噻唑衍生物是一类新的α-葡萄糖苷酶抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号