Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-03-21 , DOI: 10.1016/j.bmc.2018.03.027 Thilani M. Anthony , Mary Kay H. Pflum
|
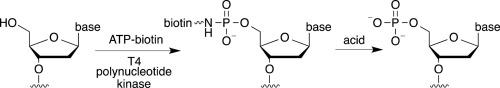
Prior work documented use of γ-phosphate modified ATP analogs to label DNA using T4 polynucleotide kinases (T4PNK), although applications have been limited. To fully characterize kinase-catalyzed labeling of nucleic acids, we explored use of ATP-biotin as a cosubstrate with T4PNK. T4PNK accepted ATP-biotin to 5′-label single stranded DNA. However, T4PNK-mediated labeling of double stranded substrates was low yielding. In addition, the phosphoramidate bond connecting the biotin group to the DNA was unstable. These results suggest that kinase-catalyzed biotinylation will be useful with single stranded DNA substrates and mild reaction conditions. By revealing the scope and limitations of kinase-catalyzed biotinylation, these studies provide a foundation for future development and application of kinase-catalyzed labeling to DNA-based biological studies.
中文翻译:

激酶催化的DNA生物素化
先前的工作记录了使用γ-磷酸修饰的ATP类似物通过T4多核苷酸激酶(T4PNK)标记DNA的过程,尽管应用受到限制。为了完全表征激酶催化的核酸标记,我们探索了将ATP-生物素与T4PNK共同用作底物的用途。T4PNK接受ATP生物素至5'-标记单链DNA。然而,T4PNK介导的双链底物标记是低产量。另外,将生物素基团连接到DNA的氨基磷酸酯键是不稳定的。这些结果表明,激酶催化的生物素化将对单链DNA底物和温和的反应条件有用。通过揭示激酶催化的生物素化的范围和局限性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号