Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2018-03-21 , DOI: 10.1016/j.apcata.2018.03.020 João F.S. de Oliveira , Diogo P. Volanti , José M.C. Bueno , Adriana P. Ferreira
|
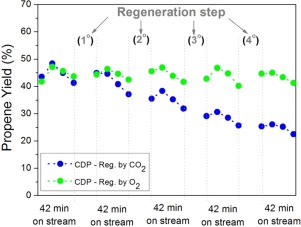
Investigation was made on the effect of chromium content, and method of hydrothermal preparation of Cr/ZrO2 catalysts on their catalytic properties for CO2 oxidative dehydrogenation of propane (ODP). The catalysts were characterized by nitrogen physisorption, X-ray diffraction, Raman spectroscopy, and temperature programmed reduction and desorption of CO2 (TPD-CO2). The Cr/ZrO2 catalysts containing various chromium contents between 2.5 and 15 wt.% of Cr were prepared by conventional and microwave-assisted hydrothermal methods. Tetragonal ZrO2 was formed, with higher Cr contents, and smaller crystallite sizes obtained using the microwave-assisted method. The relationship between selectivity to propene and propane conversion suggested that independent of the preparation method, the catalytic properties could be classified in two groups, with low and high chromium contents. The TPD-CO2 results showed that a fraction of the CO2 was desorbed at temperatures higher than 500 °C, with simultaneous reduction of Cr(VI) species. The presence of CO2 in the reactants caused strong decreases of activity, selectivity, and yield towards propene. These results suggested that CO2 adsorbed strongly on the chromium oxide active sites for dehydrogenation of propane. The Cr/ZrO2 catalysts were active and selective for dehydrogenation of propane in the absence of CO2 (CDP), and became deactivated with time on stream. The activity was reestablished by thermal treatment with CO2 or O2 after deactivation in CDP catalytic cycles, with the activity always being reestablished by treatment in O2. The deactivation occurred by reduction of Cr(VI) species and by deposition of carbonaceous species produced in oligomerization reactions.
中文翻译:

CO 2在Cr / ZrO 2催化剂上丙烷氧化脱氢反应中的作用
研究了铬含量的影响,以及水热制备Cr / ZrO 2催化剂的方法对它们催化丙烷CO 2氧化脱氢(ODP)的催化性能。催化剂的特征在于氮的物理吸附,X射线衍射,拉曼光谱和程序升温还原和脱附CO 2(TPD-CO 2)。通过常规的和微波辅助的水热方法制备了Cr含量为2.5至15wt。%的Cr / ZrO 2催化剂,其中Cr含量在2.5wt%至15wt%之间。四方ZrO 2用微波辅助法形成的铬含量较高,而晶粒尺寸较小。丙烯选择性与丙烷转化率之间的关系表明,与制备方法无关,催化性能可分为低铬含量和高铬含量两类。TPD-CO 2结果表明,一部分CO 2在高于500°C的温度下脱附,同时还原了Cr(VI)物种。反应物中CO 2的存在导致活性,选择性和向丙烯的收率大大降低。这些结果表明,CO 2强烈吸附在氧化铬活性位上,用于丙烷脱氢。Cr / ZrO 2催化剂在不存在CO 2(CDP)的情况下对丙烷脱氢具有活性和选择性,并随着生产时间的延长而失活。在CDP催化循环中失活后,通过用CO 2或O 2进行热处理来恢复活性,而在O 2中进行处理则总是恢复该活性。失活是通过还原Cr(VI)物种和沉积产生的碳质物种而发生的。在低聚反应中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号