当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrodeposition of gold on oxidized and reduced graphite surfaces and its influence on glucose oxidation
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jelechem.2018.03.037 R.A. Escalona-Villalpando , M.P. Gurrola , G. Trejo , M. Guerra-Balcázar , J. Ledesma-García , L.G. Arriaga
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jelechem.2018.03.037 R.A. Escalona-Villalpando , M.P. Gurrola , G. Trejo , M. Guerra-Balcázar , J. Ledesma-García , L.G. Arriaga
|
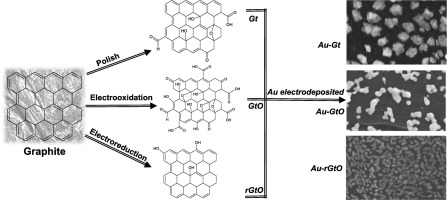
Abstract Gold surfaces have been extensively studied to achieve glucose oxidation for such applications as fuel cells or sensors. However, obtaining structures with high catalytic activity requires complex techniques for their synthesis. In this study, we propose a simple method to obtain gold architectures with high catalytic activity for glucose oxidation based on previous electrooxidation and electroreduction of graphite surfaces. The graphite (Gt) surfaces were electrochemically treated by the chronoamperometry technique obtaining graphite oxide GtO and reduced graphite oxide rGtO and these were characterized by Raman spectroscopy for determined to exhibit oxygen–containing functional groups. Subsequently, gold was electrodeposited on the previously modified graphitic surfaces by cyclic voltammetry and physicochemically characterized by SEM, XRD and AFM. XRD analysis and electrochemical profiles confirm that Au-Gt favours the presence of the [111] crystallographic plane; meanwhile, for Au-GtO, the [110] and [100] planes predominate, and finally, over rGtO, [111] and [110] are the principal planes. These surfaces exhibit catalytic activity in glucose oxidation in the order Au-GtO > Au-rGtO > Au-Gt in alkaline medium, as observed in electrochemical testing. This result demonstrates how the presence of oxygen functional groups interferes with the growth of different crystal planes, which are directly involved with the catalytic activity toward glucose oxidation.
中文翻译:

氧化还原石墨表面金的电沉积及其对葡萄糖氧化的影响
摘要 金表面已被广泛研究以实现燃料电池或传感器等应用的葡萄糖氧化。然而,获得具有高催化活性的结构需要复杂的合成技术。在这项研究中,我们提出了一种简单的方法,基于先前的石墨表面电氧化和电还原,获得对葡萄糖氧化具有高催化活性的金结构。通过计时电流法对石墨 (Gt) 表面进行电化学处理,获得氧化石墨 GtO 和还原氧化石墨 rGtO,并通过拉曼光谱对其进行表征,以确定其具有含氧官能团。随后,通过循环伏安法将金电沉积在先前改性的石墨表面上,并通过 SEM 进行物理化学表征,XRD 和 AFM。XRD 分析和电化学曲线证实 Au-Gt 有利于 [111] 晶面的存在;同时,对于 Au-GtO,[110] 和 [100] 平面占主导地位,最后,在 rGtO 之上,[111] 和 [110] 是主要平面。如电化学测试中所观察到的,这些表面在碱性介质中按照 Au-GtO > Au-rGtO > Au-Gt 的顺序在葡萄糖氧化中表现出催化活性。该结果证明了氧官能团的存在如何干扰不同晶面的生长,这些晶面直接与对葡萄糖氧化的催化活性有关。[111] 和 [110] 是主平面。如电化学测试中所观察到的,这些表面在碱性介质中按照 Au-GtO > Au-rGtO > Au-Gt 的顺序在葡萄糖氧化中表现出催化活性。该结果证明了氧官能团的存在如何干扰不同晶面的生长,这些晶面直接与对葡萄糖氧化的催化活性有关。[111] 和 [110] 是主平面。如电化学测试中所观察到的,这些表面在碱性介质中按照 Au-GtO > Au-rGtO > Au-Gt 的顺序在葡萄糖氧化中表现出催化活性。该结果证明了氧官能团的存在如何干扰不同晶面的生长,这些晶面直接与对葡萄糖氧化的催化活性有关。
更新日期:2018-05-01
中文翻译:

氧化还原石墨表面金的电沉积及其对葡萄糖氧化的影响
摘要 金表面已被广泛研究以实现燃料电池或传感器等应用的葡萄糖氧化。然而,获得具有高催化活性的结构需要复杂的合成技术。在这项研究中,我们提出了一种简单的方法,基于先前的石墨表面电氧化和电还原,获得对葡萄糖氧化具有高催化活性的金结构。通过计时电流法对石墨 (Gt) 表面进行电化学处理,获得氧化石墨 GtO 和还原氧化石墨 rGtO,并通过拉曼光谱对其进行表征,以确定其具有含氧官能团。随后,通过循环伏安法将金电沉积在先前改性的石墨表面上,并通过 SEM 进行物理化学表征,XRD 和 AFM。XRD 分析和电化学曲线证实 Au-Gt 有利于 [111] 晶面的存在;同时,对于 Au-GtO,[110] 和 [100] 平面占主导地位,最后,在 rGtO 之上,[111] 和 [110] 是主要平面。如电化学测试中所观察到的,这些表面在碱性介质中按照 Au-GtO > Au-rGtO > Au-Gt 的顺序在葡萄糖氧化中表现出催化活性。该结果证明了氧官能团的存在如何干扰不同晶面的生长,这些晶面直接与对葡萄糖氧化的催化活性有关。[111] 和 [110] 是主平面。如电化学测试中所观察到的,这些表面在碱性介质中按照 Au-GtO > Au-rGtO > Au-Gt 的顺序在葡萄糖氧化中表现出催化活性。该结果证明了氧官能团的存在如何干扰不同晶面的生长,这些晶面直接与对葡萄糖氧化的催化活性有关。[111] 和 [110] 是主平面。如电化学测试中所观察到的,这些表面在碱性介质中按照 Au-GtO > Au-rGtO > Au-Gt 的顺序在葡萄糖氧化中表现出催化活性。该结果证明了氧官能团的存在如何干扰不同晶面的生长,这些晶面直接与对葡萄糖氧化的催化活性有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号