当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization and Control of Irreversible Reaction in Li-Rich Cathode during the Initial Charge Process
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acsami.7b12722 HyeJin Lee 1 , Suk Bum Lim 2 , Jin Young Kim 2 , Mihee Jeong 1 , Yong Joon Park 2 , Won-Sub Yoon 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acsami.7b12722 HyeJin Lee 1 , Suk Bum Lim 2 , Jin Young Kim 2 , Mihee Jeong 1 , Yong Joon Park 2 , Won-Sub Yoon 1
Affiliation
|
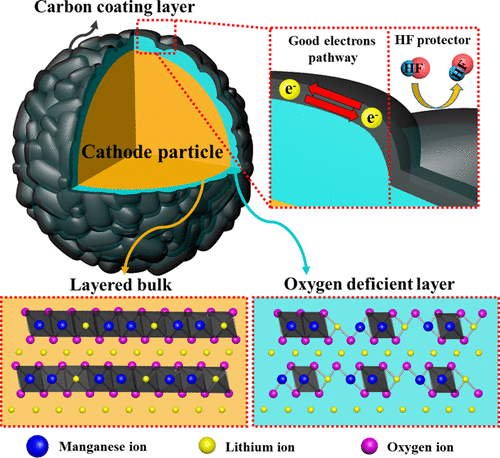
Li-rich layered oxide has been known to possess high specific capacity beyond the theoretical value from both charge compensation in transition metal and oxygen in the redox reaction. Although it could achieve higher reversible capacity due to the oxygen anion participating in electrochemical reaction, however, its use in energy storage systems has been limited. The reason is the irreversible oxygen reaction that occurs during the initial charge cycle, resulting in structural instability due to oxygen evolution and phase transition. To suppress the initial irreversible oxygen reaction, we introduced the surface-modified Li[Li0.2Ni0.16Mn0.56Co0.08]O2 prepared by carbon coating (carbonization process), which was verified to have reduced oxygen reaction during the initial charge cycle. The electrochemical performance is improved by the synergic effects of the oxygen-deficient layer and carbon coating layer formed on the surface of particles. The sample with suitable carbon coating exhibited the highest structural stability, resulting in reduced capacity fading and voltage decay, which are attributed to the mitigated layered-to-spinel-like phase transition during prolonged cycling. The control over the oxygen reaction of Li2MnO3 by surface modification affects the activation reaction above 4.4 V in the initial charge cycle and structure changes during prolonged cycling. X-ray diffraction, X-ray photoelectron spectroscopy, and X-ray absorption spectroscopy analyses as well as electrochemical performance measurement were used to identify the correlation between reduced oxygen activity and structural changes.
中文翻译:

富锂阴极初始装料过程中不可逆反应的表征和控制
从过渡金属中的电荷补偿和氧化还原反应中的氧两者来看,已知富锂的层状氧化物具有超过理论值的高比容量。尽管由于氧阴离子参与电化学反应而可以实现更高的可逆容量,但是,其在能量存储系统中的使用受到了限制。原因是在初始充电周期中发生不可逆的氧反应,由于氧的释放和相变而导致结构不稳定。为了抑制最初的不可逆的氧反应,我们引入了表面改性的Li [Li 0.2 Ni 0.16 Mn 0.56 Co 0.08 ] O 2通过碳涂层(碳化过程)制备,经验证在初始充电周期中氧反应减少。形成在颗粒表面上的缺氧层和碳涂层的协同作用改善了电化学性能。具有合适碳涂层的样品表现出最高的结构稳定性,从而降低了容量衰减和电压衰减,这归因于在延长的循环过程中缓和的层状至尖晶石状相变。Li 2 MnO 3氧反应的控制在初始充电周期中,经表面改性的表面活性剂会影响4.4 V以上的活化反应,并在延长的循环过程中改变结构。X射线衍射,X射线光电子能谱和X射线吸收能谱分析以及电化学性能测量被用来确定氧活度降低与结构变化之间的相关性。
更新日期:2018-03-21
中文翻译:

富锂阴极初始装料过程中不可逆反应的表征和控制
从过渡金属中的电荷补偿和氧化还原反应中的氧两者来看,已知富锂的层状氧化物具有超过理论值的高比容量。尽管由于氧阴离子参与电化学反应而可以实现更高的可逆容量,但是,其在能量存储系统中的使用受到了限制。原因是在初始充电周期中发生不可逆的氧反应,由于氧的释放和相变而导致结构不稳定。为了抑制最初的不可逆的氧反应,我们引入了表面改性的Li [Li 0.2 Ni 0.16 Mn 0.56 Co 0.08 ] O 2通过碳涂层(碳化过程)制备,经验证在初始充电周期中氧反应减少。形成在颗粒表面上的缺氧层和碳涂层的协同作用改善了电化学性能。具有合适碳涂层的样品表现出最高的结构稳定性,从而降低了容量衰减和电压衰减,这归因于在延长的循环过程中缓和的层状至尖晶石状相变。Li 2 MnO 3氧反应的控制在初始充电周期中,经表面改性的表面活性剂会影响4.4 V以上的活化反应,并在延长的循环过程中改变结构。X射线衍射,X射线光电子能谱和X射线吸收能谱分析以及电化学性能测量被用来确定氧活度降低与结构变化之间的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号