当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Regeneration of Chirality with l-Cysteine through 1,3-Dipolar Cycloadditions between Diazoalkanes and Enantiomerically Pure Thiazolines: Experimental and Computational Studies
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.joc.8b00312 J. Gracia-Vitoria 1 , I. Osante 1 , C. Cativiela 1 , P. Merino 2 , T. Tejero 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.joc.8b00312 J. Gracia-Vitoria 1 , I. Osante 1 , C. Cativiela 1 , P. Merino 2 , T. Tejero 1
Affiliation
|
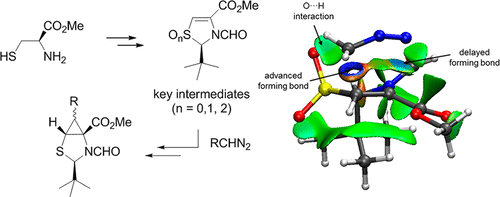
Enantiopure 2-thia-4-azabicyclo[3.1.0]hexanes, which can be considered constrained cysteines, have been obtained from l-cysteine by application of the “self-regeneration of chirality” concept. The key intermediates are homochiral thiazolines that can be prepared in multigram scale and react smoothly with a series of diazoalkanes providing Δ1-pyrazolines except for phenyldiazomethane that yield the isomeric Δ2-pyrazolines. Nitrogen extrusion in Δ1-pyrazolines and further reduction of the sulfinyl group yielded the target compounds in good overall yield. Computational studies of the cycloaddition reaction were used for determining the polarity of the process and explaining the observed stereoselectivity. Additional topological studies were employed for determining the influence of noncovalent interactions in the stereochemical course of the reaction, which showed to be a highly asynchronous concerted process.
中文翻译:

通过重氮烷烃和对映体纯的噻唑啉类之间的1,3-偶极环加成反应,用半胱氨酸自动再生手性:实验和计算研究
对映体纯的2-thia-4-azabicyclo [3.1.0]己烷,可以被认为是受限制的半胱氨酸,是通过应用“手性的自我再生”概念从l-半胱氨酸中获得的。关键中间体是可以在多克规模制备,并且具有一系列重氮提供Δ的顺利反应的纯手性噻唑啉1 -pyrazolines除了phenyldiazomethane能产生同分异构的Δ 2个-pyrazolines。在Δ氮气挤出1-吡唑啉和亚磺酰基的进一步还原以良好的总收率得到目标化合物。对环加成反应的计算研究用于确定过程的极性并解释观察到的立体选择性。其他拓扑研究用于确定反应的立体化学过程中非共价相互作用的影响,这表明这是一个高度异步的协同过程。
更新日期:2018-03-14
中文翻译:

通过重氮烷烃和对映体纯的噻唑啉类之间的1,3-偶极环加成反应,用半胱氨酸自动再生手性:实验和计算研究
对映体纯的2-thia-4-azabicyclo [3.1.0]己烷,可以被认为是受限制的半胱氨酸,是通过应用“手性的自我再生”概念从l-半胱氨酸中获得的。关键中间体是可以在多克规模制备,并且具有一系列重氮提供Δ的顺利反应的纯手性噻唑啉1 -pyrazolines除了phenyldiazomethane能产生同分异构的Δ 2个-pyrazolines。在Δ氮气挤出1-吡唑啉和亚磺酰基的进一步还原以良好的总收率得到目标化合物。对环加成反应的计算研究用于确定过程的极性并解释观察到的立体选择性。其他拓扑研究用于确定反应的立体化学过程中非共价相互作用的影响,这表明这是一个高度异步的协同过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号