当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorination increases the electron mobility of zinc azadipyrromethene-based electron acceptors and enhances the performance of fullerene-free organic solar cells†
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c7tc05820a Sandra Pejić 1, 2, 3, 4 , Anna M. Thomsen 1, 2, 3, 4 , Forrest S. Etheridge 1, 2, 3, 4 , Roshan Fernando 1, 2, 3, 4 , Chunlai Wang 1, 2, 3, 4 , Geneviève Sauvé 1, 2, 3, 4
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c7tc05820a Sandra Pejić 1, 2, 3, 4 , Anna M. Thomsen 1, 2, 3, 4 , Forrest S. Etheridge 1, 2, 3, 4 , Roshan Fernando 1, 2, 3, 4 , Chunlai Wang 1, 2, 3, 4 , Geneviève Sauvé 1, 2, 3, 4
Affiliation
|
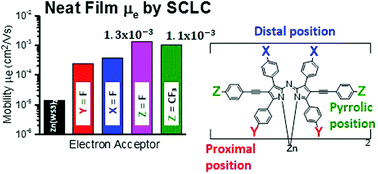
Structure–property studies were performed on a series of four fluorinated zinc azadipyrromethene derivatives. This series is based on bis[2,8-diphenylethynyl-1,3,7,9-tetraphenylazadipyrromethene]zinc(II) (Zn(WS3)2), with fluorine atoms at the para-position of either the proximal phenyls, the distal phenyls or the pyrrolic phenylethynyls, refered to as the proximal, distal and pyrrolic positions, respectively. In order to study the degree of fluorination, we also added –CF3 to the pyrrolic positions. All complexes had similar absorption spectra, 600–800 nm in films, complementing the absorption of the well-known donor poly(3-hexylthiophene) (P3HT). The chelates were tested in bulk heterojunction organic photovoltaic (OPV) devices using P3HT as the electron donor. Compared to the unfluorinated acceptor Zn(WS3)2, fluorination increased the power conversion efficiencies (PCEs) in all cases except in the proximal position. The best results were obtained when either F or CF3 was added to the pyrrolic positions with PCEs of 3.3% and 3.7%. Atomic force microscopy images revealed a favorable phase separation and showed no signs of large-scale aggregation for all blends. Light intensity measurements revealed that bimolecular recombination limits the performance in these fullerene-free devices, and that the addition of fluorine suppressed the bimolecular recombination, with the largest suppression seen with the pyrrolic substitutions F and CF3. Electron mobility increased with fluorination, again with the largest increase when fluorines were added to the pyrrolic positions, reaching mobilities as high as ∼10−3 cm2 V−1 s−1, on a par with electron mobility of the ubiquitous phenyl-C61-butyric acid methyl ester (PCBM) acceptor in blends. These results point to the importance of the chemical composition of pyrrolic phenylethylnyls for optimizing the charge transport and device performance for these types of complexes.
中文翻译:

氟化提高了基于氮杂二吡咯锌锌的电子受体的电子迁移率,并增强了不含富勒烯的有机太阳能电池的性能†
对一系列四种氟化锌氮杂二吡咯亚甲基衍生物进行了结构性质研究。该系列基于双[2,8-二苯基乙炔基-1,3,7,9-四苯基叠氮二吡咯亚甲基] zinc(II)(Zn(WS3)2),在近端苯基的对位上有氟原子,远端苯基或吡咯基苯基乙炔基,分别称为近端,远端和吡咯基。为了研究氟化程度,我们还添加了–CF 3到吡咯位置。所有复合物在膜中的吸收光谱相似,在600–800 nm之间,与众所周知的供体聚(3-己基噻吩)(P3HT)的吸收互补。使用P3HT作为电子供体,在体异质结有机光伏(OPV)器件中测试了螯合物。与未氟化的受体Zn(WS3)2相比,氟化在所有情况下都提高了功率转换效率(PCE),除了近端位置。当F或CF 3时,可获得最佳结果添加到吡咯位置,PCE分别为3.3%和3.7%。原子力显微镜图像显示出有利的相分离,并且没有显示出所有混合物的大规模聚集的迹象。光强度测量表明,双分子重组限制了这些不含富勒烯的装置的性能,并且氟的添加抑制了双分子重组,其中最大的抑制作用见于吡咯取代基F和CF 3。电子迁移率随氟化作用而增加,当氟被添加到吡咯位置时,迁移率又增加最大,达到高达〜10 -3 cm 2 V -1 s -1的迁移率,与普遍存在的苯基-C的电子迁移率相当共混物中的61-丁酸甲酯(PCBM)受体。这些结果表明,对于优化这些类型的配合物的电荷传输和器件性能,吡咯基苯基乙腈的化学组成很重要。
更新日期:2018-03-21
中文翻译:

氟化提高了基于氮杂二吡咯锌锌的电子受体的电子迁移率,并增强了不含富勒烯的有机太阳能电池的性能†
对一系列四种氟化锌氮杂二吡咯亚甲基衍生物进行了结构性质研究。该系列基于双[2,8-二苯基乙炔基-1,3,7,9-四苯基叠氮二吡咯亚甲基] zinc(II)(Zn(WS3)2),在近端苯基的对位上有氟原子,远端苯基或吡咯基苯基乙炔基,分别称为近端,远端和吡咯基。为了研究氟化程度,我们还添加了–CF 3到吡咯位置。所有复合物在膜中的吸收光谱相似,在600–800 nm之间,与众所周知的供体聚(3-己基噻吩)(P3HT)的吸收互补。使用P3HT作为电子供体,在体异质结有机光伏(OPV)器件中测试了螯合物。与未氟化的受体Zn(WS3)2相比,氟化在所有情况下都提高了功率转换效率(PCE),除了近端位置。当F或CF 3时,可获得最佳结果添加到吡咯位置,PCE分别为3.3%和3.7%。原子力显微镜图像显示出有利的相分离,并且没有显示出所有混合物的大规模聚集的迹象。光强度测量表明,双分子重组限制了这些不含富勒烯的装置的性能,并且氟的添加抑制了双分子重组,其中最大的抑制作用见于吡咯取代基F和CF 3。电子迁移率随氟化作用而增加,当氟被添加到吡咯位置时,迁移率又增加最大,达到高达〜10 -3 cm 2 V -1 s -1的迁移率,与普遍存在的苯基-C的电子迁移率相当共混物中的61-丁酸甲酯(PCBM)受体。这些结果表明,对于优化这些类型的配合物的电荷传输和器件性能,吡咯基苯基乙腈的化学组成很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号