当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A bi-terminal protein ligation strategy to probe chromatin structure during DNA damage†
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8sc00681d Sinan Kilic 1, 2, 3, 4, 5 , Iuliia Boichenko 1, 2, 3, 4, 5 , Carolin C. Lechner 1, 2, 3, 4, 5 , Beat Fierz 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8sc00681d Sinan Kilic 1, 2, 3, 4, 5 , Iuliia Boichenko 1, 2, 3, 4, 5 , Carolin C. Lechner 1, 2, 3, 4, 5 , Beat Fierz 1, 2, 3, 4, 5
Affiliation
|
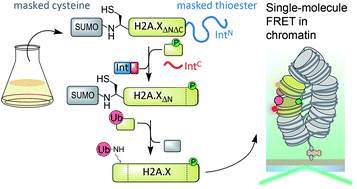
The cellular response to DNA damage results in a signaling cascade that primes chromatin for repair. Combinatorial post-translational modifications (PTMs) play an important role in this process by altering the physical properties of chromatin and recruiting downstream factors. One key signal integrator is the histone variant H2A.X, which is phosphorylated at a C-terminal serine (S139ph), and ubiquitylated within its N-terminal tail at lysines 13 and 15 (K13/15ub). How these PTMs directly impact chromatin structure and thereby facilitate DNA repair is not well understood. Detailed studies require synthetic access to such N- and C-terminally modified proteins. This is complicated by the requirement for protecting groups allowing multi-fragment assembly. Here, we report a semi-synthetic route to generate simultaneously N- and C-terminally modified proteins using genetically encoded orthogonal masking groups. Applied to H2A.X, expression of a central protein fragment, containing a protected N-terminal cysteine and a C-terminal thioester masked as a split intein, enables sequential C- and N-terminal protein modification and results in the convergent production of H2A.X carrying K15ub and S139ph. Using single-molecule FRET between defined nucleosomes in synthetic chromatin fibers, we then show that K15 ubiquitylation (but not S139ph) impairs nucleosome stacking in tetranucleosome units, opening chromatin during DNA repair.
中文翻译:

在DNA损伤期间探测染色质结构的双末端蛋白质连接策略†
细胞对DNA损伤的反应导致信号传导级联,引发染色质修复。组合翻译后修饰(PTM)通过改变染色质的物理特性和募集下游因子在此过程中发挥重要作用。一个关键的信号整合者是组蛋白变体H2A.X,其在C端丝氨酸(S139ph)处被磷酸化,并在其N端尾巴的赖氨酸13和15(K13 / 15ub)处泛素化。这些PTM如何直接影响染色质结构,从而促进DNA修复尚不清楚。详细的研究要求合成获得此类N和C末端修饰的蛋白质。由于需要保护基团以允许多片段组装,这使情况变得复杂。这里,我们报告了使用遗传编码的正交掩蔽基团同时生成N和C末端修饰的蛋白质的半合成路线。应用于H2A.X,中央蛋白片段的表达,包含受保护的N端半胱氨酸和被掩盖为分裂内含蛋白的C端硫酯,可实现连续的C端和N端蛋白修饰,并导致H2A的融合生产.X带有K15ub和S139ph。然后,使用合成染色质纤维中定义的核小体之间的单分子FRET,我们显示K15泛素化(而不是S139ph)损害了四核小体单元中的核小体堆积,从而在DNA修复过程中打开了染色质。含有受保护的N端半胱氨酸和被掩盖为分裂内含蛋白的C端硫酯,可实现连续的C端和N端蛋白修饰,并最终产生携带K15ub和S139ph的H2A.X。然后,使用合成染色质纤维中定义的核小体之间的单分子FRET,我们显示K15泛素化(而不是S139ph)损害了四核小体单元中的核小体堆积,从而在DNA修复过程中打开了染色质。含有受保护的N端半胱氨酸和被掩盖为分裂内含蛋白的C端硫酯,可实现连续的C端和N端蛋白修饰,并最终产生携带K15ub和S139ph的H2A.X。然后,使用合成染色质纤维中定义的核小体之间的单分子FRET,我们显示K15泛素化(而不是S139ph)损害了四核小体单元中的核小体堆积,从而在DNA修复过程中打开了染色质。
更新日期:2018-03-21
中文翻译:

在DNA损伤期间探测染色质结构的双末端蛋白质连接策略†
细胞对DNA损伤的反应导致信号传导级联,引发染色质修复。组合翻译后修饰(PTM)通过改变染色质的物理特性和募集下游因子在此过程中发挥重要作用。一个关键的信号整合者是组蛋白变体H2A.X,其在C端丝氨酸(S139ph)处被磷酸化,并在其N端尾巴的赖氨酸13和15(K13 / 15ub)处泛素化。这些PTM如何直接影响染色质结构,从而促进DNA修复尚不清楚。详细的研究要求合成获得此类N和C末端修饰的蛋白质。由于需要保护基团以允许多片段组装,这使情况变得复杂。这里,我们报告了使用遗传编码的正交掩蔽基团同时生成N和C末端修饰的蛋白质的半合成路线。应用于H2A.X,中央蛋白片段的表达,包含受保护的N端半胱氨酸和被掩盖为分裂内含蛋白的C端硫酯,可实现连续的C端和N端蛋白修饰,并导致H2A的融合生产.X带有K15ub和S139ph。然后,使用合成染色质纤维中定义的核小体之间的单分子FRET,我们显示K15泛素化(而不是S139ph)损害了四核小体单元中的核小体堆积,从而在DNA修复过程中打开了染色质。含有受保护的N端半胱氨酸和被掩盖为分裂内含蛋白的C端硫酯,可实现连续的C端和N端蛋白修饰,并最终产生携带K15ub和S139ph的H2A.X。然后,使用合成染色质纤维中定义的核小体之间的单分子FRET,我们显示K15泛素化(而不是S139ph)损害了四核小体单元中的核小体堆积,从而在DNA修复过程中打开了染色质。含有受保护的N端半胱氨酸和被掩盖为分裂内含蛋白的C端硫酯,可实现连续的C端和N端蛋白修饰,并最终产生携带K15ub和S139ph的H2A.X。然后,使用合成染色质纤维中定义的核小体之间的单分子FRET,我们显示K15泛素化(而不是S139ph)损害了四核小体单元中的核小体堆积,从而在DNA修复过程中打开了染色质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号