当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A definitive assessment of the CO oxidation pattern of a nanocomposite MnCeOx catalyst†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8re00026c Francesco Arena 1, 2, 3, 4, 5 , Roberto di Chio 1, 2, 3, 4 , Claudia Espro 1, 2, 3, 4 , Alessandra Palella 4, 5, 6 , Lorenzo Spadaro 4, 5, 6
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8re00026c Francesco Arena 1, 2, 3, 4, 5 , Roberto di Chio 1, 2, 3, 4 , Claudia Espro 1, 2, 3, 4 , Alessandra Palella 4, 5, 6 , Lorenzo Spadaro 4, 5, 6
Affiliation
|
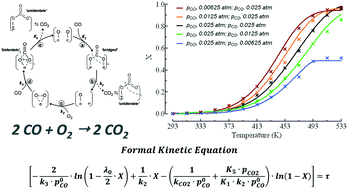
The CO oxidation pattern of a nanocomposite MnCeOx catalyst (M5C1; Mnat/Ceat, 5) in the range of 293–533 K (P, 1 atm) has been probed under a kinetic regime, varying reagent pressure (p0CO, 0.01–0.025 atm; λ0, 1), 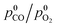 ratio (λ0, 0.25–4.0) and CO2 co-feeding (0.05–0.10 atm). Activity data indicate fractional orders on pCO (0.6 ± 0.1) and pO2 (0.4 ± 0.1), with an activation energy of 40 ± 3 kJ mol−1, and a negative kinetic effect of CO2 co-feeding due to competitive adsorption processes. Coupled with systematic evidence on the reactivity and mobility of catalyst oxygen and surface intermediates, kinetic data disclose a concerted redox mechanism of Langmuir–Hinshelwood type, which starts by abstraction of O-atoms from surface active MnIV centres (r.d.s.), and is sustained by adsorption of diatomic oxygen species on O-vacancies. The derivative and integral forms of the formal rate equation explain the empirical kinetics, predicting the activity pattern of the MnCeOx catalyst in the range of 293–533 K.
ratio (λ0, 0.25–4.0) and CO2 co-feeding (0.05–0.10 atm). Activity data indicate fractional orders on pCO (0.6 ± 0.1) and pO2 (0.4 ± 0.1), with an activation energy of 40 ± 3 kJ mol−1, and a negative kinetic effect of CO2 co-feeding due to competitive adsorption processes. Coupled with systematic evidence on the reactivity and mobility of catalyst oxygen and surface intermediates, kinetic data disclose a concerted redox mechanism of Langmuir–Hinshelwood type, which starts by abstraction of O-atoms from surface active MnIV centres (r.d.s.), and is sustained by adsorption of diatomic oxygen species on O-vacancies. The derivative and integral forms of the formal rate equation explain the empirical kinetics, predicting the activity pattern of the MnCeOx catalyst in the range of 293–533 K.
中文翻译:

纳米复合MnCeO x催化剂的CO氧化模式的权威评估†
纳米复合材料的MnCeO CO氧化图案X催化剂;(M5C1的Mn在/ CE在在293-533 K(的范围内,5)P,1个大气压)已被动力学制度下探测,不同试剂压力(p 0 CO,0.01-0.025大气压; λ 0,1),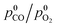 比率(λ 0,0.25-4.0)和CO 2共进料(0.05-0.10大气压)。活性数据表明在p CO(0.6±0.1)和p O 2(0.4±0.1)上的分数阶,活化能为40±3 kJ mol -1,并且CO 2的动力学效应为负由于竞争性吸附过程而共同进料。结合有关催化剂氧和表面中间体的反应性和迁移率的系统证据,动力学数据揭示了Langmuir-Hinshelwood型的协同氧化还原机理,该机理始于从表面活性Mn IV中心(rds)提取O原子,并持续存在。通过在O空位上吸附双原子氧物种。正规速率方程的导数和积分形式解释了实证动力学,预测MnCeO的活动模式X在293-533 K的范围的催化剂
比率(λ 0,0.25-4.0)和CO 2共进料(0.05-0.10大气压)。活性数据表明在p CO(0.6±0.1)和p O 2(0.4±0.1)上的分数阶,活化能为40±3 kJ mol -1,并且CO 2的动力学效应为负由于竞争性吸附过程而共同进料。结合有关催化剂氧和表面中间体的反应性和迁移率的系统证据,动力学数据揭示了Langmuir-Hinshelwood型的协同氧化还原机理,该机理始于从表面活性Mn IV中心(rds)提取O原子,并持续存在。通过在O空位上吸附双原子氧物种。正规速率方程的导数和积分形式解释了实证动力学,预测MnCeO的活动模式X在293-533 K的范围的催化剂
更新日期:2018-03-21
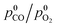 ratio (λ0, 0.25–4.0) and CO2 co-feeding (0.05–0.10 atm). Activity data indicate fractional orders on pCO (0.6 ± 0.1) and pO2 (0.4 ± 0.1), with an activation energy of 40 ± 3 kJ mol−1, and a negative kinetic effect of CO2 co-feeding due to competitive adsorption processes. Coupled with systematic evidence on the reactivity and mobility of catalyst oxygen and surface intermediates, kinetic data disclose a concerted redox mechanism of Langmuir–Hinshelwood type, which starts by abstraction of O-atoms from surface active MnIV centres (r.d.s.), and is sustained by adsorption of diatomic oxygen species on O-vacancies. The derivative and integral forms of the formal rate equation explain the empirical kinetics, predicting the activity pattern of the MnCeOx catalyst in the range of 293–533 K.
ratio (λ0, 0.25–4.0) and CO2 co-feeding (0.05–0.10 atm). Activity data indicate fractional orders on pCO (0.6 ± 0.1) and pO2 (0.4 ± 0.1), with an activation energy of 40 ± 3 kJ mol−1, and a negative kinetic effect of CO2 co-feeding due to competitive adsorption processes. Coupled with systematic evidence on the reactivity and mobility of catalyst oxygen and surface intermediates, kinetic data disclose a concerted redox mechanism of Langmuir–Hinshelwood type, which starts by abstraction of O-atoms from surface active MnIV centres (r.d.s.), and is sustained by adsorption of diatomic oxygen species on O-vacancies. The derivative and integral forms of the formal rate equation explain the empirical kinetics, predicting the activity pattern of the MnCeOx catalyst in the range of 293–533 K.
中文翻译:

纳米复合MnCeO x催化剂的CO氧化模式的权威评估†
纳米复合材料的MnCeO CO氧化图案X催化剂;(M5C1的Mn在/ CE在在293-533 K(的范围内,5)P,1个大气压)已被动力学制度下探测,不同试剂压力(p 0 CO,0.01-0.025大气压; λ 0,1),
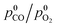 比率(λ 0,0.25-4.0)和CO 2共进料(0.05-0.10大气压)。活性数据表明在p CO(0.6±0.1)和p O 2(0.4±0.1)上的分数阶,活化能为40±3 kJ mol -1,并且CO 2的动力学效应为负由于竞争性吸附过程而共同进料。结合有关催化剂氧和表面中间体的反应性和迁移率的系统证据,动力学数据揭示了Langmuir-Hinshelwood型的协同氧化还原机理,该机理始于从表面活性Mn IV中心(rds)提取O原子,并持续存在。通过在O空位上吸附双原子氧物种。正规速率方程的导数和积分形式解释了实证动力学,预测MnCeO的活动模式X在293-533 K的范围的催化剂
比率(λ 0,0.25-4.0)和CO 2共进料(0.05-0.10大气压)。活性数据表明在p CO(0.6±0.1)和p O 2(0.4±0.1)上的分数阶,活化能为40±3 kJ mol -1,并且CO 2的动力学效应为负由于竞争性吸附过程而共同进料。结合有关催化剂氧和表面中间体的反应性和迁移率的系统证据,动力学数据揭示了Langmuir-Hinshelwood型的协同氧化还原机理,该机理始于从表面活性Mn IV中心(rds)提取O原子,并持续存在。通过在O空位上吸附双原子氧物种。正规速率方程的导数和积分形式解释了实证动力学,预测MnCeO的活动模式X在293-533 K的范围的催化剂









































 京公网安备 11010802027423号
京公网安备 11010802027423号