当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1α/NF-κB signaling pathway
Food & Function ( IF 5.1 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c7fo01406f Juan Chen 1, 2, 3, 4, 5 , Minghua Zhang 4, 6, 7, 8 , Maomao Zhu 4, 5, 9, 10, 11 , Junfei Gu 4, 5, 9, 10 , Jie Song 4, 5, 9, 10 , Li Cui 4, 5, 9, 10 , Dan Liu 4, 5, 9, 10 , Qing Ning 4, 5, 9, 10 , Xiaobin Jia 4, 12, 13, 14 , Liang Feng 4, 12, 13, 14
Food & Function ( IF 5.1 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c7fo01406f Juan Chen 1, 2, 3, 4, 5 , Minghua Zhang 4, 6, 7, 8 , Maomao Zhu 4, 5, 9, 10, 11 , Junfei Gu 4, 5, 9, 10 , Jie Song 4, 5, 9, 10 , Li Cui 4, 5, 9, 10 , Dan Liu 4, 5, 9, 10 , Qing Ning 4, 5, 9, 10 , Xiaobin Jia 4, 12, 13, 14 , Liang Feng 4, 12, 13, 14
Affiliation
|
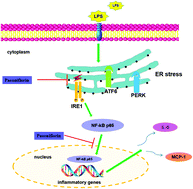
Endoplasmic reticulum (ER) stress-associated inflammation is a critical molecular mechanism involved in the pathogenesis of endothelial dysfunction (ED). Hence, strategies for alleviating ER stress-induced inflammation may be essential for the prevention of cardiovascular diseases. Paeoniflorin (PF), a bioactive compound from Paeonia lactiflora Pallas is known for its functional properties against vascular inflammation. However, to date, PF-mediated protection against ER stress-dependent inflammation has not been identified. Herein, we investigate the protective effect of PF on lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cell (HUVEC) injury and explore its underlying mechanism. The result of the cell viability assay indicates that PF promotes the cell survival rate in LPS-stimulated HUVECs. In addition, the LPS-induced over-production of inflammatory cytokines (interleukin-6 (IL-6) and monocyte chemotactic protein 1 (MCP-1)) and ER stress markers (78 kDa glucose regulated protein (GRP78) and CCAAT/enhancer binding protein homologous protein (CHOP)) are significantly decreased by PF and the ER stress inhibitor 4-phenylbutric acid (4-PBA). The transmission electron microscopy (TEM) assay implies that the ultrastructural abnormalities in ER are reversed by PF treatment, which is similar to the protective effect of 4-PBA. Impressively, we find that the inositol-requiring enzyme 1α (IRE1α)/nuclear factor-kappa B (NF-κB) pathway is significantly activated and contributes to the progress of LPS-induced HUVEC injury by promoting inflammatory cytokine production. IRE1α siRNA, AEBSF (ATF6 inhibitor), GSK2656157 (PERK inhibitor), PDTC (NF-κB inhibitor) and thapsigargin (TG, IRE1 activator) are used to confirm the role of the IRE1α/NF-κB pathway in PF-mediated protection against LPS-induced HUVEC injury. Our findings indicate that PF has an inhibitory effect on endothelial injury. To summarize, PF might be a potential therapeutic agent to inhibit ER stress-associated vascular inflammation.
中文翻译:

eon药苷通过IRE1α/NF-κB信号通路 防止脂多糖刺激的人脐静脉内皮细胞内质网应激相关的炎症
内质网(ER)应激相关炎症是内皮功能障碍(ED)发病机理中的关键分子机制。因此,减轻ER应激引起的炎症的策略对于预防心血管疾病可能是必不可少的。eon药(PF),一种来自Pa药的生物活性化合物Pallas以其抗血管炎症的功能特性而闻名。然而,迄今为止,尚未确定PF介导的针对ER应激依赖性炎症的保护作用。在这里,我们调查PF对脂多糖(LPS)刺激的人脐静脉内皮细胞(HUVEC)损伤的保护作用,并探讨其潜在机制。细胞活力测定的结果表明,PF促进了LPS刺激的HUVECs的细胞存活率。此外,LPS诱导的炎症细胞因子(白介素6(IL-6)和单核细胞趋化蛋白1(MCP-1))和ER应激标志物(78 kDa葡萄糖调节蛋白(GRP78)和CCAAT /增强子)的过量生产PF和ER应激抑制剂4-苯基丁酸(4-PBA)显着降低了结合蛋白同源蛋白(CHOP)的含量。透射电子显微镜(TEM)分析表明,通过PF处理可以逆转ER中的超微结构异常,这与4-PBA的保护作用相似。令人印象深刻的是,我们发现需要肌醇的酶1α(IRE1α)/核因子-κB(NF-κB)途径被显着激活,并通过促进炎症性细胞因子的产生而促进LPS诱导的HUVEC损伤的进展。IRE1αsiRNA,AEBSF(ATF6抑制剂),GSK2656157(PERK抑制剂),PDTC(NF-κB抑制剂)和毒胡萝卜素(TG,IRE1激活剂)用于确认IRE1α/NF-κB途径在PF介导的针对PF的保护中的作用。 LPS诱导的HUVEC损伤。我们的发现表明PF对内皮损伤具有抑制作用。综上所述,PF可能是抑制ER应激相关血管炎症的潜在治疗剂。
更新日期:2018-03-21
中文翻译:

eon药苷通过IRE1α/NF-κB信号通路 防止脂多糖刺激的人脐静脉内皮细胞内质网应激相关的炎症
内质网(ER)应激相关炎症是内皮功能障碍(ED)发病机理中的关键分子机制。因此,减轻ER应激引起的炎症的策略对于预防心血管疾病可能是必不可少的。eon药(PF),一种来自Pa药的生物活性化合物Pallas以其抗血管炎症的功能特性而闻名。然而,迄今为止,尚未确定PF介导的针对ER应激依赖性炎症的保护作用。在这里,我们调查PF对脂多糖(LPS)刺激的人脐静脉内皮细胞(HUVEC)损伤的保护作用,并探讨其潜在机制。细胞活力测定的结果表明,PF促进了LPS刺激的HUVECs的细胞存活率。此外,LPS诱导的炎症细胞因子(白介素6(IL-6)和单核细胞趋化蛋白1(MCP-1))和ER应激标志物(78 kDa葡萄糖调节蛋白(GRP78)和CCAAT /增强子)的过量生产PF和ER应激抑制剂4-苯基丁酸(4-PBA)显着降低了结合蛋白同源蛋白(CHOP)的含量。透射电子显微镜(TEM)分析表明,通过PF处理可以逆转ER中的超微结构异常,这与4-PBA的保护作用相似。令人印象深刻的是,我们发现需要肌醇的酶1α(IRE1α)/核因子-κB(NF-κB)途径被显着激活,并通过促进炎症性细胞因子的产生而促进LPS诱导的HUVEC损伤的进展。IRE1αsiRNA,AEBSF(ATF6抑制剂),GSK2656157(PERK抑制剂),PDTC(NF-κB抑制剂)和毒胡萝卜素(TG,IRE1激活剂)用于确认IRE1α/NF-κB途径在PF介导的针对PF的保护中的作用。 LPS诱导的HUVEC损伤。我们的发现表明PF对内皮损伤具有抑制作用。综上所述,PF可能是抑制ER应激相关血管炎症的潜在治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号