当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coordination ability of amino acid hydrazide ligands and their influence on magnetic properties in copper(ii) coordination polymers†
CrystEngComm ( IF 2.6 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8ce00210j Lidija Androš Dubraja 1, 2, 3, 4, 5 , Ivanka Jerić 2, 3, 4, 6 , Andreas Puškarić 1, 2, 3, 4 , Josip Bronić 1, 2, 3, 4 , Eufemio Moreno-Pineda 5, 7, 8, 9
CrystEngComm ( IF 2.6 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8ce00210j Lidija Androš Dubraja 1, 2, 3, 4, 5 , Ivanka Jerić 2, 3, 4, 6 , Andreas Puškarić 1, 2, 3, 4 , Josip Bronić 1, 2, 3, 4 , Eufemio Moreno-Pineda 5, 7, 8, 9
Affiliation
|
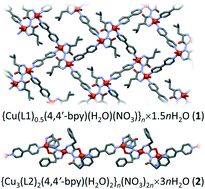
Newly synthesized amino acid hydrazide ligands (L1 = N,N′-di-L-leucine hydrazide; L2 = N,N′-di-L-phenylalanine hydrazide) have shown excellent reactivity and stability, as well as extraordinary coordination ability. Reactions of L1/L2 with copper(II) ions and 4,4′-bipyridine (4,4′-bpy) as a secondary spacer yielded new coordination polymers {Cu(L1)0.5(4,4′-bpy)(H2O)(NO3)}n·1.5nH2O (1) and {Cu3(L2)2(4,4′-bpy)(H2O)2}n(NO3)2n·3nH2O (2). UV/vis spectroscopy together with structural analysis showed that the deprotonated ligands L12−/L22− with six available donor atoms per ligand have the ability for strong chelation to copper(II) ions, acting as bis-bidentate (L12−) and bridging bi + tridentate (L22−) ligands. Compound 1 contains copper(II) ions simultaneously bridged with short hydrazide bridges (4.725 Å) and long 4,4′-bpy spacers (11.144 Å) forming a two-dimensional coordination network. In compound 2, a pair of L22− molecules bridges three copper(II) ions, which are further interconnected with 4,4′-bpy into an overall one-dimensional chain-like structure. Although bulkier, the phenylalanine ligand (L2) was found to be more flexible compared to the leucine ligand (L1), exhibiting additional intramolecular contact: cation–π interaction. In both compounds, the hydrazide (–N–N–) bridge mediates the antiferromagnetic interaction between copper(II) ions. Hydrogen bonds have a strong influence on the crystal packing and physical (magnetic) properties. The reported compounds are first examples of hydrazide-bridged metal centres featuring amino acid-based ligands.
中文翻译:

氨基酸酰肼配体的配位能力及其对铜(ii)配位聚合物的磁性的影响†
新合成的氨基酸酰肼配体(L1 = N,N'-二-L-亮氨酸酰肼;L2 = N,N'-二-L-苯丙氨酸酰肼)显示出优异的反应性和稳定性,以及出色的配位能力。的反应L1 / L2与铜(II)离子和4,4'-联吡啶(4,4'-联吡啶)作为二次隔离物,得到新的配位聚合物{的Cu(L1)0.5(4,4'-联吡啶)(H 2 O)(NO 3)} n ·1.5 n H 2 O(1)和{Cu 3(L2)2(4,4'-bpy)(H 2 O)2 } n(NO 3)2 n ·3 n H 2 O(2)。紫外/可见光谱和结构分析表明,每个配体具有六个可用供体原子的去质子化的配体L1 2- / L2 2-具有强螯合铜(II)离子的能力,可作为双双齿(L1 2-)。和桥接bi +三齿(L2 2-)配体。化合物1含有铜(II)离子同时用短的酰肼桥(4.725Å)和长的4,4'-bpy间隔基(11.144Å)桥接,形成二维配位网络。在化合物2中,一对L2 2-分子桥接三个铜(II)离子,它们进一步与4,4'-bpy互连成整体一维链状结构。尽管体积更大,但发现苯丙氨酸配体(L2)比亮氨酸配体(L1)更灵活,表现出额外的分子内接触:阳离子-π相互作用。在这两种化合物中,酰肼(–N–N–)桥介导铜之间的反铁磁相互作用(II)离子。氢键对晶体堆积和物理(磁性)性能有很大影响。报道的化合物是具有基于氨基酸的配体的酰肼桥接的金属中心的第一个实例。
更新日期:2018-03-21
中文翻译:

氨基酸酰肼配体的配位能力及其对铜(ii)配位聚合物的磁性的影响†
新合成的氨基酸酰肼配体(L1 = N,N'-二-L-亮氨酸酰肼;L2 = N,N'-二-L-苯丙氨酸酰肼)显示出优异的反应性和稳定性,以及出色的配位能力。的反应L1 / L2与铜(II)离子和4,4'-联吡啶(4,4'-联吡啶)作为二次隔离物,得到新的配位聚合物{的Cu(L1)0.5(4,4'-联吡啶)(H 2 O)(NO 3)} n ·1.5 n H 2 O(1)和{Cu 3(L2)2(4,4'-bpy)(H 2 O)2 } n(NO 3)2 n ·3 n H 2 O(2)。紫外/可见光谱和结构分析表明,每个配体具有六个可用供体原子的去质子化的配体L1 2- / L2 2-具有强螯合铜(II)离子的能力,可作为双双齿(L1 2-)。和桥接bi +三齿(L2 2-)配体。化合物1含有铜(II)离子同时用短的酰肼桥(4.725Å)和长的4,4'-bpy间隔基(11.144Å)桥接,形成二维配位网络。在化合物2中,一对L2 2-分子桥接三个铜(II)离子,它们进一步与4,4'-bpy互连成整体一维链状结构。尽管体积更大,但发现苯丙氨酸配体(L2)比亮氨酸配体(L1)更灵活,表现出额外的分子内接触:阳离子-π相互作用。在这两种化合物中,酰肼(–N–N–)桥介导铜之间的反铁磁相互作用(II)离子。氢键对晶体堆积和物理(磁性)性能有很大影响。报道的化合物是具有基于氨基酸的配体的酰肼桥接的金属中心的第一个实例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号