Chemosphere ( IF 8.1 ) Pub Date : 2018-03-20 , DOI: 10.1016/j.chemosphere.2018.03.117 Agnieszka Cuprys , Rama Pulicharla , Joanna Lecka , Satinder Kaur Brar , Patrick Drogui , R.Y. Surampalli
|
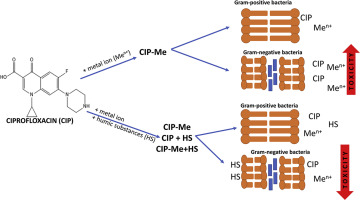
The co-contamination of ciprofloxacin (CIP) with metal ions results in alteration of CIP mobility, antimicrobial activity and distribution/development of the antibiotic-resistance genes. In this study, the stability of five CIP-Me complexes [Me = Al(III), Co(II), Cu(II), Fe(III), Mg] was investigated in the presence of humic substances (HS) at two temperatures 18 ± 2 °C and 4 ± 1 °C for seven days period. The most stable complexes were CIP-Al, CIP-Cu, and CIP-Co with the stability constants (K) at 18 °C 35.5 ± 1.4 11.5 ± 1.5 and 11.7 ± 1.5 respectively. At lower temperature (4 °C), the stability constants decreased: 1-fold for CIP-Al, 14-fold for CIP-Co and 2-fold for CIP-Cu. The presence of humic substances decreased the stability of complexes. The chemical reactions of Fe3+ in water at circumneutral pH resulted in stability alteration. The formation of CIP-Mg complexes at lower temperatures and in the presence of HS was limited. In ultrapure water, CIP-Me complexes exhibit higher toxicity towards Gram-negative Enterobacter aeruginosa (ranged between 0.125 and 0.5 μg/ml). However, the presence of HS reduced the antimicrobial activity of CIP-Me complexes by at least 2-fold. Gram-positive representative, Bacillus subtilis was not affected by the presence of metal ions and/or HS. The toxicity toward B. subtilis for the complexes was equal to toxicity of CIP alone (MIC = 0.25 μg/ml). This suggested the different susceptibility to CIP and its complexes.
中文翻译:

环丙沙星金属配合物–在腐殖质存在下的稳定性和毒性测试
环丙沙星(CIP)与金属离子的共污染会导致CIP迁移率,抗菌活性和抗生素抗性基因的分布/发育发生变化。在这项研究中,研究了五种CIP-Me配合物[Me = Al(III),Co(II),Cu(II),Fe(III),Mg]在两种腐殖质(HS)存在下的稳定性。温度为18±2°C和4±1°C,持续7天。最稳定的配合物是CIP-Al,CIP-Cu和CIP-Co,在18°C时的稳定性常数(K)分别为35.5±1.4 11.5±1.5和11.7±1.5。在较低温度(4°C)下,稳定性常数降低:CIP-Al为1倍,CIP-Co为14倍,CIP-Cu为2倍。腐殖质的存在降低了复合物的稳定性。Fe 3+的化学反应pH值在水中的水会导致稳定性改变。在较低的温度和HS存在下,CIP-Mg复合物的形成受到限制。在超纯水中,CIP-Me复合物对革兰氏阴性铜绿肠杆菌显示更高的毒性(范围在0.125至0.5μg/ ml之间)。但是,HS的存在使CIP-Me复合物的抗微生物活性降低了至少2倍。革兰氏阳性代表,枯草芽孢杆菌不受金属离子和/或HS的影响。复合物对枯草芽孢杆菌的毒性等于单独的CIP毒性(MIC = 0.25μg/ ml)。这表明对CIP及其复合物的敏感性不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号