Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-03-19 , DOI: 10.1016/j.jcat.2018.02.021 Mi Wan , Decheng Liang , Li Wang , Xiangwen Zhang , Dong Yang , Guozhu Li
|
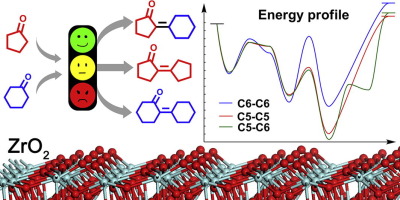
Aldol condensation of cycloketone(s) is an efficient reaction path to synthesize polycyclic compounds via CC bond formation. In this work, aldol condensation of cyclopentanone and/or cyclohexanone over monoclinic ZrO2 has been investigated by combination of experiments and DFT calculations. Both self-condensation and cross-condensation of cyclopentanone and/or cyclohexanone were carried out at 130 °C in a solvent-free condition under atmospheric pressure. Reactant selectivity and product distribution were analyzed. DFT calculations were performed to investigate the mechanism and reactant selectivity of cycloketone condensation on ZrO2, showing the favored cross-condensation between cyclopentanone and cyclohexanone is ascribed to the formation of a metastable state of a seven-membered ring that lowers activation barrier during C
C bond formation.
中文翻译:

氧化锆催化的环酮缩合反应:反应物选择性的起源
环酮的醛醇缩合是通过C C键形成合成多环化合物的有效反应途径。在这项工作中,通过实验和DFT计算的结合,研究了环戊酮和/或环己酮在单斜ZrO 2上的羟醛缩合反应。环戊酮和/或环己酮的自缩合和交叉缩合均在130°C,无溶剂条件下,大气压下进行。分析了反应物的选择性和产物分布。进行了DFT计算以研究环酮缩合在ZrO 2上的机理和反应物的选择性图7显示环戊酮和环己酮之间有利的交叉缩合归因于形成七元环的亚稳态,该环在CC
键形成期间降低了活化势垒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号