Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.jssc.2018.03.010 O.V. Merkulov , A.A. Markov , I.A. Leonidov , M.V. Patrakeev , V.L. Kozhevnikov
|
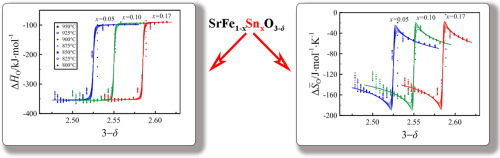
The oxygen content (3–δ) variations in tin substituted derivatives SrFe1–xSnxO3–δ, where x = 0.05, 0.1, 0.17 and 0.25, of perovskite-like strontium ferrite, have been studied by coulometric titration measurements within oxygen partial pressure (pO2) range 10–19–10–2 atm at 800–950 °С. The obtained dependencies of (3–δ ) from pO2 and temperature are used for calculations of partial molar thermodynamic functions of oxygen in the oxide structure. It is found that a satisfactory explanation of the experimental results can be attained within frameworks of the ideal solution model with ion and electron defects appearing in the result of oxidation and disproportionation of iron cations. The increase of the oxidation reaction enthalpy with tin content is consistent with the increase of the unit cell parameter, i.e., the stretch and relaxation of Fe-O chemical bonds.
中文翻译:

固溶体SrFe 1– x Sn x O 3– δ中的氧非化学计量和热力学量
通过库仑滴定法研究了锡取代的钙钛矿状锶铁氧体中SrFe 1– x Sn x O 3– δ(其中x = 0.05、0.1、0.17和0.25 )中的氧含量(3- δ)变化。氧分压(p O 2)在800-950°С的范围内10 –19 –10 –2 atm。从p O 2获得的(3 –δ)依赖性使用温度和温度来计算氧在氧化物结构中的部分摩尔热力学函数。发现在理想溶液模型的框架内可以获得令人满意的实验结果解释,其中离子和电子缺陷是由于铁阳离子的氧化和歧化而出现的。氧化反应焓随锡含量的增加与晶胞参数(即Fe-O化学键的拉伸和弛豫)的增加一致。









































 京公网安备 11010802027423号
京公网安备 11010802027423号