当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The impact of O-glycan chemistry on the stability of intrinsically disordered proteins†
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1039/c7sc05016j Erica T Prates 1, 2 , Xiaoyang Guan 3 , Yaohao Li 3 , Xinfeng Wang 3 , Patrick K Chaffey 3 , Munir S Skaf 2 , Michael F Crowley 4 , Zhongping Tan 3 , Gregg T Beckham 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1039/c7sc05016j Erica T Prates 1, 2 , Xiaoyang Guan 3 , Yaohao Li 3 , Xinfeng Wang 3 , Patrick K Chaffey 3 , Munir S Skaf 2 , Michael F Crowley 4 , Zhongping Tan 3 , Gregg T Beckham 1
Affiliation
|
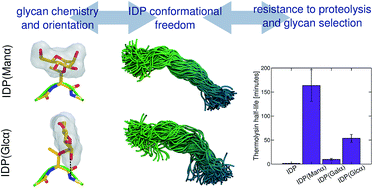
Protein glycosylation is a diverse post-translational modification that serves myriad biological functions. O-linked glycans in particular vary widely in extent and chemistry in eukaryotes, with secreted proteins from fungi and yeast commonly exhibiting O-mannosylation in intrinsically disordered regions of proteins, likely for proteolysis protection, among other functions. However, it is not well understood why mannose is often the preferred glycan, and more generally, if the neighboring protein sequence and glycan have coevolved to protect against proteolysis in glycosylated intrinsically disordered proteins (IDPs). Here, we synthesized variants of a model IDP, specifically a natively O-mannosylated linker from a fungal enzyme, with α-O-linked mannose, glucose, and galactose moieties, along with a non-glycosylated linker. Upon exposure to thermolysin, O-mannosylation, by far, provides the highest extent of proteolysis protection. To explain this observation, extensive molecular dynamics simulations were conducted, revealing that the axial configuration of the C2-hydroxyl group (2-OH) of α-mannose adjacent to the glycan–peptide bond strongly influences the conformational features of the linker. Specifically, α-mannose restricts the torsions of the IDP main chain more than other glycans whose equatorial 2-OH groups exhibit interactions that favor perpendicular glycan–protein backbone orientation. We suggest that IDP stiffening due to O-mannosylation impairs protease action, with contributions from protein–glycan interactions, protein flexibility, and protein stability. Our results further imply that resistance to proteolysis is an important driving force for evolutionary selection of α-mannose in eukaryotic IDPs, and more broadly, that glycan motifs for proteolysis protection likely coevolve with the protein sequence to which they attach.
中文翻译:

O-聚糖化学对本质无序蛋白质稳定性的影响†
蛋白质糖基化是一种多样化的翻译后修饰,具有多种生物学功能。尤其是真核生物中, O-连接聚糖的程度和化学性质差异很大,来自真菌和酵母的分泌蛋白通常在蛋白质的本质无序区域中表现出O-甘露糖基化,可能用于蛋白水解保护等功能。然而,目前尚不清楚为什么甘露糖通常是首选聚糖,更一般地说,是否相邻的蛋白质序列和聚糖共同进化以防止糖基化本质无序蛋白质(IDP)中的蛋白水解。在这里,我们合成了模型IDP的变体,特别是来自真菌酶的天然O-甘露糖基化接头,具有α- O-连接的甘露糖、葡萄糖和半乳糖部分,以及非糖基化接头。到目前为止,接触嗜热菌蛋白酶后,O-甘露糖基化可提供最高程度的蛋白水解保护。为了解释这一观察结果,进行了广泛的分子动力学模拟,揭示了与聚糖-肽键相邻的 α-甘露糖的 C2-羟基 (2-OH) 的轴向构型强烈影响连接子的构象特征。具体而言,α-甘露糖比其他聚糖更能限制 IDP 主链的扭转,其赤道 2-OH 基团表现出有利于垂直聚糖-蛋白质主链方向的相互作用。我们认为, O-甘露糖基化导致的 IDP 硬化会损害蛋白酶的作用,其中蛋白质-聚糖相互作用、蛋白质灵活性和蛋白质稳定性都有贡献。我们的结果进一步表明,对蛋白水解的抗性是真核 IDP 中 α-甘露糖进化选择的重要驱动力,更广泛地说,用于蛋白水解保护的聚糖基序可能与它们所附着的蛋白质序列共同进化。
更新日期:2018-03-20
中文翻译:

O-聚糖化学对本质无序蛋白质稳定性的影响†
蛋白质糖基化是一种多样化的翻译后修饰,具有多种生物学功能。尤其是真核生物中, O-连接聚糖的程度和化学性质差异很大,来自真菌和酵母的分泌蛋白通常在蛋白质的本质无序区域中表现出O-甘露糖基化,可能用于蛋白水解保护等功能。然而,目前尚不清楚为什么甘露糖通常是首选聚糖,更一般地说,是否相邻的蛋白质序列和聚糖共同进化以防止糖基化本质无序蛋白质(IDP)中的蛋白水解。在这里,我们合成了模型IDP的变体,特别是来自真菌酶的天然O-甘露糖基化接头,具有α- O-连接的甘露糖、葡萄糖和半乳糖部分,以及非糖基化接头。到目前为止,接触嗜热菌蛋白酶后,O-甘露糖基化可提供最高程度的蛋白水解保护。为了解释这一观察结果,进行了广泛的分子动力学模拟,揭示了与聚糖-肽键相邻的 α-甘露糖的 C2-羟基 (2-OH) 的轴向构型强烈影响连接子的构象特征。具体而言,α-甘露糖比其他聚糖更能限制 IDP 主链的扭转,其赤道 2-OH 基团表现出有利于垂直聚糖-蛋白质主链方向的相互作用。我们认为, O-甘露糖基化导致的 IDP 硬化会损害蛋白酶的作用,其中蛋白质-聚糖相互作用、蛋白质灵活性和蛋白质稳定性都有贡献。我们的结果进一步表明,对蛋白水解的抗性是真核 IDP 中 α-甘露糖进化选择的重要驱动力,更广泛地说,用于蛋白水解保护的聚糖基序可能与它们所附着的蛋白质序列共同进化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号