当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Investigation on Ni-Catalyzed C(sp3)–F Activation and Ring Contraction of Tetrahydropyrans: Exploration of an SN2 Pathway
Organometallics ( IF 2.5 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00894 Zheyuan Xu 1 , Yinuo Yang 1 , Julong Jiang 1 , Yao Fu 1
Organometallics ( IF 2.5 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00894 Zheyuan Xu 1 , Yinuo Yang 1 , Julong Jiang 1 , Yao Fu 1
Affiliation
|
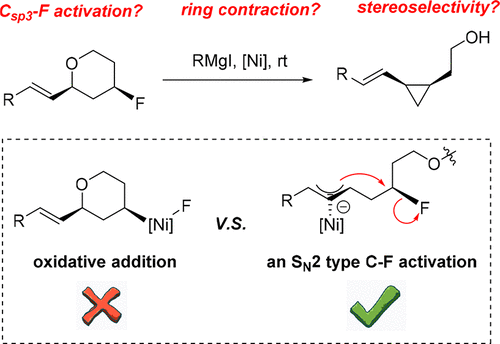
DFT calculations were conducted to elucidate the mechanistic details of a recently reported cross-electrophile coupling (i.e., XEC) reaction, in which a stable C(sp3)–F bond was successfully cleaved and a ring contraction from six-membered tetrahydropyran to three-membered cyclopropane was realized. Our theoretical investigation highlights the critical role that the Grignard reagents play. Despite being a reducing agent, the RMgX also helps to promote the C(sp3)–O cleavage while breaking the tetrahydropyran ring and to activate the C(sp3)–F bond to furnish the final recyclization. The cleavage of the C(sp3)–F bond follows an SN2-type reaction, so that a chirality inversion of the carbon can be ensured. Regarding the details of the cross-coupling between two electrophiles, the pathway involving radicals is not possible for this system. According to our calculations, this reaction in fact follows an unconventional Tsuji–Trost reaction, which means that, rather than being attacked by a nucleophile, the terminal carbon of the allyl group performs a nucleophilic attack by itself. More specifically, it is realized by a methyl transfer from the RMgX to the Ni atom (i.e., methylation on the Ni atom), which is almost barrierless. In addition, the recyclization leading to the formation of cyclopropane is followed by another methyl migration, which affords a NiMe2 species. The NiMe2 species then undergoes a reductive elimination to regenerate the Ni(0) catalyst with a release of ethane. Meanwhile, the reductive elimination, rather than the recyclization process as proposed, is found to be the rate-determining step with an effective free energy barrier of only 69.8 kJ/mol, which ensures the reaction to proceed smoothly even at room temperature.
中文翻译:

镍催化四氢吡喃的C(sp 3)–F活化和环收缩的理论研究:S N 2途径的探索
进行DFT计算是为了阐明最近报道的交叉亲电子偶联(即XEC)反应的机理细节,该反应成功地裂解了稳定的C(sp 3)-F键,并使环从六元四氢吡喃缩合为三个元环丙烷得以实现。我们的理论研究突出了格氏试剂的关键作用。尽管是还原剂,RMgX还有助于促进C(sp 3)-O裂解,同时打破四氢吡喃环并激活C(sp 3)-F键以提供最终的再循环。C(sp 3)–F键的裂解遵循S N2型反应,从而可以确保碳的手性反转。关于两个亲电试剂之间的交叉偶联的细节,涉及自由基的途径对于该系统是不可能的。根据我们的计算,该反应实际上是在非常规的Tsuji-Trost反应之后进行的,这意味着烯丙基的末端碳本身不受亲核体的攻击,而不是受到亲核体的攻击。更具体地,这是通过从RMgX到Ni原子的甲基转移(即,Ni原子上的甲基化)实现的,这几乎是无障碍的。另外,导致环丙烷形成的再循环随后是另一种甲基迁移,这提供了NiMe 2物质。NiMe 2然后对这些物种进行还原消除,以释放出乙烷,从而再生Ni(0)催化剂。同时,发现还原消除而不是提议的再循环过程是速率决定步骤,有效自由能垒仅为69.8 kJ / mol,即使在室温下也能确保反应顺利进行。
更新日期:2018-03-20
中文翻译:

镍催化四氢吡喃的C(sp 3)–F活化和环收缩的理论研究:S N 2途径的探索
进行DFT计算是为了阐明最近报道的交叉亲电子偶联(即XEC)反应的机理细节,该反应成功地裂解了稳定的C(sp 3)-F键,并使环从六元四氢吡喃缩合为三个元环丙烷得以实现。我们的理论研究突出了格氏试剂的关键作用。尽管是还原剂,RMgX还有助于促进C(sp 3)-O裂解,同时打破四氢吡喃环并激活C(sp 3)-F键以提供最终的再循环。C(sp 3)–F键的裂解遵循S N2型反应,从而可以确保碳的手性反转。关于两个亲电试剂之间的交叉偶联的细节,涉及自由基的途径对于该系统是不可能的。根据我们的计算,该反应实际上是在非常规的Tsuji-Trost反应之后进行的,这意味着烯丙基的末端碳本身不受亲核体的攻击,而不是受到亲核体的攻击。更具体地,这是通过从RMgX到Ni原子的甲基转移(即,Ni原子上的甲基化)实现的,这几乎是无障碍的。另外,导致环丙烷形成的再循环随后是另一种甲基迁移,这提供了NiMe 2物质。NiMe 2然后对这些物种进行还原消除,以释放出乙烷,从而再生Ni(0)催化剂。同时,发现还原消除而不是提议的再循环过程是速率决定步骤,有效自由能垒仅为69.8 kJ / mol,即使在室温下也能确保反应顺利进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号