当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Osteogenic nanostructured titanium surfaces with antibacterial properties under conditions that mimic the dynamic situation in the oral cavity†
Biomaterials Science ( IF 5.8 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1039/c8bm00177d Susanne Bierbaum 1, 2, 3, 4, 5 , Susan Mulansky 1, 3, 4, 6 , Eszter Bognár 7, 8, 9, 10, 11 , Imre Kientzl 7, 8, 9, 10, 11 , Péter Nagy 7, 8, 9, 10, 11 , Nihal Engin Vrana 12, 13, 14 , Miklós Weszl 11, 15, 16, 17, 18 , Elke Boschke 1, 3, 4, 6 , Dieter Scharnweber 1, 2, 3, 4 , Cornelia Wolf-Brandstetter 1, 2, 3, 4
Biomaterials Science ( IF 5.8 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1039/c8bm00177d Susanne Bierbaum 1, 2, 3, 4, 5 , Susan Mulansky 1, 3, 4, 6 , Eszter Bognár 7, 8, 9, 10, 11 , Imre Kientzl 7, 8, 9, 10, 11 , Péter Nagy 7, 8, 9, 10, 11 , Nihal Engin Vrana 12, 13, 14 , Miklós Weszl 11, 15, 16, 17, 18 , Elke Boschke 1, 3, 4, 6 , Dieter Scharnweber 1, 2, 3, 4 , Cornelia Wolf-Brandstetter 1, 2, 3, 4
Affiliation
|
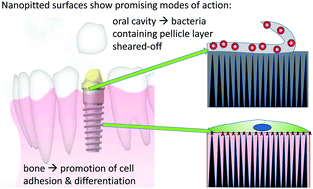
The study aim was to assess the impact of different surface nanofeatures on otherwise smooth titanium surfaces on bacterial adhesion as well as on their osteogenic potential. Bacterial adhesion was assessed in the presence of saliva under static and dynamic conditions to approximate both sub- and supragingival conditions in the oral cavity as the gingival seal will be affected by implantation. The ultimate goal was to develop a surface that will reduce biofilm formation but still support osseointegration in vivo. To this end nanotubular or nanopitted surfaces were created on electropolished titanium via electrochemical anodization procedures. Sandblasted/acid etched surfaces (SBAE) were used as a microrough reference. Bacterial adhesion was studied using saliva-precoated samples with S. sanguinis as a typical early colonizer of the oral cavity; osteogenic differentiation was assessed with human bone marrow stromal cells. While bacterial adhesion was reduced on all microsmooth surfaces to an average of 17% surface coverage compared to 61% on SBAE under static conditions, under dynamic conditions the nanopitted surface had a significant impact on bacterial adhesion. Here fluid flow removed all bacteria. By comparison, the reduction on the nanotubular surface was only similar to that of the SBAE reference. We hypothesise the underlying cause to be an effect of the surface morphology on the structure and composition of the saliva precoating that reduces its stability, giving rise to a self-cleaning effect. In addition, no negative influence on the osteogenic potential of the nanopitted surface could be determined by alkaline phosphatase activity, mineralization behaviour or gene expression; it remained on a par with the tissue culture plastic control. Thus, nanopitting seems to be a promising surface treatment candidate for dental implants to reduce infection related complications without compromising the implant integration.
中文翻译:

在模拟口腔中动态情况的条件下,成骨纳米结构钛表面具有抗菌性能†
该研究的目的是评估不同表面纳米特征对原本光滑的钛表面对细菌粘附及其成骨潜能的影响。在静态和动态条件下,在唾液存在的情况下评估细菌的粘附力,以近似于牙龈下和龈上条件,因为牙龈密封会受到植入的影响。最终目的是开发一种表面,该表面将减少生物膜的形成,但仍支持体内骨整合。为此,通过电化学阳极氧化程序在电抛光的钛上形成纳米管或纳米点蚀的表面。喷砂/酸蚀表面(SBAE)用作微粗糙参考。使用唾液包被的样品研究细菌粘附桑吉氏葡萄球菌作为口腔的典型早期定居者;用人骨髓基质细胞评估成骨分化。虽然在静态条件下所有微光滑表面上的细菌粘附力平均降低到17%,而在SBAE上平均只有61%,但在动态条件下,纳米点蚀的表面对细菌粘附力有显着影响。在这里,流体流清除了所有细菌。相比之下,纳米管表面的减少仅与SBAE参考的减少相似。我们假设根本原因是表面形态对唾液预涂层结构和成分的影响,从而降低了其稳定性,从而产生了自清洁效果。此外,碱性磷酸酶活性,矿化行为或基因表达不能确定对纳米点蚀表面成骨潜力的负面影响;它与组织培养塑料对照品保持一致。因此,纳米点蚀似乎是用于牙科植入物的有希望的表面处理候选物,以减少感染相关的并发症而不损害植入物的整合。
更新日期:2018-03-20
中文翻译:

在模拟口腔中动态情况的条件下,成骨纳米结构钛表面具有抗菌性能†
该研究的目的是评估不同表面纳米特征对原本光滑的钛表面对细菌粘附及其成骨潜能的影响。在静态和动态条件下,在唾液存在的情况下评估细菌的粘附力,以近似于牙龈下和龈上条件,因为牙龈密封会受到植入的影响。最终目的是开发一种表面,该表面将减少生物膜的形成,但仍支持体内骨整合。为此,通过电化学阳极氧化程序在电抛光的钛上形成纳米管或纳米点蚀的表面。喷砂/酸蚀表面(SBAE)用作微粗糙参考。使用唾液包被的样品研究细菌粘附桑吉氏葡萄球菌作为口腔的典型早期定居者;用人骨髓基质细胞评估成骨分化。虽然在静态条件下所有微光滑表面上的细菌粘附力平均降低到17%,而在SBAE上平均只有61%,但在动态条件下,纳米点蚀的表面对细菌粘附力有显着影响。在这里,流体流清除了所有细菌。相比之下,纳米管表面的减少仅与SBAE参考的减少相似。我们假设根本原因是表面形态对唾液预涂层结构和成分的影响,从而降低了其稳定性,从而产生了自清洁效果。此外,碱性磷酸酶活性,矿化行为或基因表达不能确定对纳米点蚀表面成骨潜力的负面影响;它与组织培养塑料对照品保持一致。因此,纳米点蚀似乎是用于牙科植入物的有希望的表面处理候选物,以减少感染相关的并发症而不损害植入物的整合。










































 京公网安备 11010802027423号
京公网安备 11010802027423号