PLOS ONE ( IF 2.9 ) Pub Date : 2018-03-19 , DOI: 10.1371/journal.pone.0194485 Candice Brinkmeyer-Langford 1 , Candice Chu 2 , Cynthia Balog-Alvarez 1 , Xue Yu 2 , James J Cai 1 , Mary Nabity 2 , Joe N Kornegay 1, 2
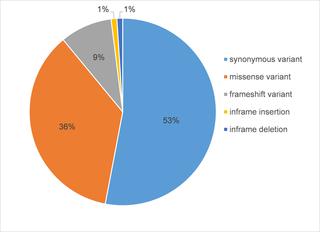
|
Duchenne muscular dystrophy (DMD) causes progressive disability in 1 of every 5,000 boys due to the lack of functional dystrophin protein. Despite much advancement in knowledge about DMD disease presentation and progression—attributable in part to studies using mouse and canine models of the disease–current DMD treatments are not equally effective in all patients. There remains, therefore, a need for translational animal models in which novel treatment targets can be identified and evaluated. Golden Retriever muscular dystrophy (GRMD) is a phenotypically and genetically homologous animal model of DMD. As with DMD, speed of disease progression in GRMD varies substantially. However, unlike DMD, all GRMD dogs possess the same causal mutation; therefore genetic modifiers of phenotypic variation are relatively easier to identify. Furthermore, the GRMD dogs used in this study reside within the same colony, reducing the confounding effects of environment on phenotypic variation. To detect modifiers of disease progression, we developed gene expression profiles using RNA sequencing for 9 dogs: 6 GRMD dogs (3 with faster-progressing and 3 with slower-progressing disease, based on quantitative, objective biomarkers) and 3 control dogs from the same colony. All dogs were evaluated at 2 time points: early disease onset (3 months of age) and the point at which GRMD stabilizes (6 months of age) using quantitative, objective biomarkers identified as robust against the effects of relatedness/inbreeding. Across all comparisons, the most differentially expressed genes fell into 3 categories: myogenesis/muscle regeneration, metabolism, and inflammation. Our findings are largely in concordance with DMD and mouse model studies, reinforcing the utility of GRMD as a translational model. Novel findings include the strong up-regulation of chitinase 3-like 1 (CHI3L1) in faster-progressing GRMD dogs, suggesting previously unexplored mechanisms underlie progression speed in GRMD and DMD. In summary, our findings support the utility of RNA sequencing for evaluating potential biomarkers of GRMD progression speed, and are valuable for identifying new avenues of exploration in DMD research.
中文翻译:

在杜兴氏肌营养不良犬模型中疾病进展的表达谱。
由于缺乏功能性肌营养不良蛋白,Duchenne肌营养不良症(DMD)导致每5,000名男孩中有1名进行性残疾。尽管有关DMD疾病表现和进展的知识有了很大的进步(部分归因于使用该疾病的小鼠和犬类模型进行的研究),但当前DMD治疗并非对所有患者都同样有效。因此,仍然需要能够识别和评估新的治疗靶标的转化动物模型。金毛寻回犬肌肉营养不良(GRMD)是DMD的表型和遗传同源动物模型。与DMD一样,GRMD中疾病进展的速度也有很大差异。但是,与DMD不同,所有GRMD狗都具有相同的因果突变。因此,表型变异的遗传修饰子相对容易识别。此外,该研究中使用的GRMD狗居住在同一殖民地内,从而减少了环境对表型变异的混杂影响。为了检测疾病进展的调节剂,我们使用RNA测序为9只狗开发了基因表达谱:6只GRMD狗(3只疾病进展快,3只疾病进展慢,基于定量的客观生物标记)和3只相同的对照狗殖民地。在两个时间点对所有犬进行评估:疾病的早期发作(3个月大)和GRMD稳定的点(6个月大),使用定量的,客观的生物标记物,这些标记物对相关性/近交的影响很强。在所有比较中,表达差异最大的基因分为3类:肌发生/肌肉再生,代谢和炎症。我们的发现在很大程度上与DMD和小鼠模型研究相一致,从而增强了GRMD作为转换模型的效用。新发现包括在进展更快的GRMD狗中几丁质酶3样1(CHI3L1)的强烈上调,表明GRMD和DMD的进展速度是先前尚未探索的机制。总而言之,我们的发现支持RNA测序在评估GRMD进程速度的潜在生物标志物方面的实用性,对于在DMD研究中确定新的探索途径具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号