当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of petal-like δ-MnO2 and its catalytic ozonation performance†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8nj00240a Kai Luo 1, 2, 3, 4, 5 , Shi-Xi Zhao 1, 2, 3, 4 , Yi-Feng Wang 1, 2, 3, 4, 5 , Shu-Jin Zhao 1, 2, 3, 4, 5 , Xi-Hui Zhang 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8nj00240a Kai Luo 1, 2, 3, 4, 5 , Shi-Xi Zhao 1, 2, 3, 4 , Yi-Feng Wang 1, 2, 3, 4, 5 , Shu-Jin Zhao 1, 2, 3, 4, 5 , Xi-Hui Zhang 1, 2, 3, 4
Affiliation
|
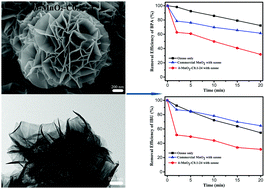
Petal-like δ-MnO2 microspheres were successfully synthesized by a very simple hydrothermal method with potassium permanganate (KMnO4) as the only raw material. By adjusting the initial concentration of KMnO4 solution and the time of the hydrothermal reaction, the morphology and crystallinity of δ-MnO2 can be controlled. The synthesized δ-MnO2 microspheres were characterized by XRD, SEM, TEM, TG/DSC, BET, ICP-AES and XPS. The catalytic ozonation performance of δ-MnO2 prepared by a hydrothermal reaction at 160 °C for 24 h using 0.1 mol L−1 KMnO4 solution was tested. Its catalytic activity is excellent. The degradation efficiencies of bisphenol A and ibuprofen using 0.1 g L−1 δ-MnO2 as the catalyst were 68.2% and 68.5% in 20 min reaction time. The results were much better than those of individual ozone treatment and with commercial MnO2 as the catalyst. The formation of hydroxyl radicals was not the main reason for the rapid degradation of organic compounds in the catalytic reaction with δ-MnO2 as the catalyst. The strong interaction among the catalyst surface, ozone and organic molecules was the important reason for catalysis based on the related experimental results.
中文翻译:

合成花瓣状δ-的MnO 2和臭氧催化氧化性能†
花瓣状δ-的MnO 2个微球成功地用高锰酸钾一个非常简单的水热法(合成的KMnO 4)作为唯一的原料。通过调整的高锰酸钾的初始浓度4溶液δ-的MnO和水热反应时,形态和结晶度2可以被控制。合成δ-的MnO 2用XRD,SEM,TEM,TG / DSC,BET,ICP-AES和XPS的微球进行了表征。δ-的MnO的催化臭氧性能2使用0.1摩尔L请在160℃下进行水热反应24小时制备-1的KMnO 4解决方案进行了测试。它的催化活性极好。双酚A和布洛芬使用0.1克L-降解效率-1 δ-的MnO 2作为催化剂分别为68.2%和20分钟反应时间68.5%。结果要好于单独的臭氧处理和以商业MnO 2为催化剂的结果。羟基自由基的形成不是用于与δ-MnO的催化反应的有机化合物的迅速降解的主要原因2作为催化剂。根据相关的实验结果,催化剂表面,臭氧和有机分子之间的强相互作用是催化的重要原因。
更新日期:2018-03-19
中文翻译:

合成花瓣状δ-的MnO 2和臭氧催化氧化性能†
花瓣状δ-的MnO 2个微球成功地用高锰酸钾一个非常简单的水热法(合成的KMnO 4)作为唯一的原料。通过调整的高锰酸钾的初始浓度4溶液δ-的MnO和水热反应时,形态和结晶度2可以被控制。合成δ-的MnO 2用XRD,SEM,TEM,TG / DSC,BET,ICP-AES和XPS的微球进行了表征。δ-的MnO的催化臭氧性能2使用0.1摩尔L请在160℃下进行水热反应24小时制备-1的KMnO 4解决方案进行了测试。它的催化活性极好。双酚A和布洛芬使用0.1克L-降解效率-1 δ-的MnO 2作为催化剂分别为68.2%和20分钟反应时间68.5%。结果要好于单独的臭氧处理和以商业MnO 2为催化剂的结果。羟基自由基的形成不是用于与δ-MnO的催化反应的有机化合物的迅速降解的主要原因2作为催化剂。根据相关的实验结果,催化剂表面,臭氧和有机分子之间的强相互作用是催化的重要原因。










































 京公网安备 11010802027423号
京公网安备 11010802027423号