当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Band Engineering of Carbon Nitride Monolayers by N-Type, P-Type, and Isoelectronic Doping for Photocatalytic Applications
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acsami.8b01729 Meysam Makaremi 1 , Sean Grixti 1 , Keith T. Butler 2 , Geoffrey A. Ozin 3 , Chandra Veer Singh 1, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acsami.8b01729 Meysam Makaremi 1 , Sean Grixti 1 , Keith T. Butler 2 , Geoffrey A. Ozin 3 , Chandra Veer Singh 1, 4
Affiliation
|
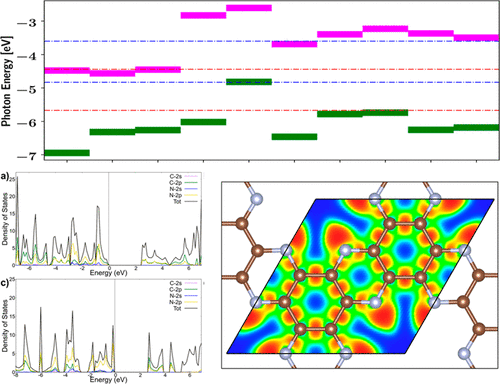
Since hydrogen fuel involves the highest energy density among all fuels, production of this gas through the solar water splitting approach has been suggested as a green remedy for greenhouse environmental issues due to extensive consumption of fossil fuels. Low-dimensional materials possessing a large surface-to-volume ratio can be a promising candidate to be used for the photocatalytic approach. Here, we used extensive first-principles calculations to investigate the application of newly fabricated members of two-dimensional carbon nitrides including tg-C3N4, hg-C3N4, C2N, and C3N for water splitting. Band engineering via N-type, P-type, and isoelectronic doping agents such as B, N, P, Si, and Ge was demonstrated for tuning the electronic structure, optimizing solar absorption and band alignment for photocatalysis. Pristine tg-C3N4, hg-C3N4, and C2N crystals involve bandgaps of 3.190, 2.772, and 2.465 eV, respectively, which are not proper for water splitting. Among the dopants, Si and Ge dopants can narrow the band gap of carbon nitrides about 0.5–1.0 eV and also increase their optical absorption in the visible spectrum. This study presents the potential for doping with isoelectronic elements to greatly improve the photocatalytic characteristics of carbon nitride nanostructures.
中文翻译:

用于光催化应用的N型,P型和等电子掺杂的碳氮化物单分子层的能带工程
由于氢燃料在所有燃料中具有最高的能量密度,因此由于化石燃料的大量消耗,已提出通过太阳能水分解法生产这种气体作为温室环境问题的绿色补救措施。具有大的表面体积比的低维材料可能是用于光催化方法的有前途的候选材料。在这里,我们使用了大量的第一性原理计算来研究二维碳氮化物的新制造成员的应用,包括tg -C 3 N 4,hg -C 3 N 4,C 2 N和C 3N用于水分解。演示了通过N型,P型和等电子掺杂剂(例如B,N,P,Si和Ge)进行能带工程,以调节电子结构,优化光吸收和光催化的能带排列。原始的tg -C 3 N 4,hg -C 3 N 4和C 2N晶体的带隙分别为3.190、2.772和2.465 eV,这不适合进行水分解。在这些掺杂剂中,Si和Ge掺杂剂可使碳氮化物的带隙变窄约0.5-1.0 eV,并在可见光谱中增加其光吸收。这项研究表明掺杂等电子元素的潜力,可以大大改善氮化碳纳米结构的光催化特性。
更新日期:2018-03-19
中文翻译:

用于光催化应用的N型,P型和等电子掺杂的碳氮化物单分子层的能带工程
由于氢燃料在所有燃料中具有最高的能量密度,因此由于化石燃料的大量消耗,已提出通过太阳能水分解法生产这种气体作为温室环境问题的绿色补救措施。具有大的表面体积比的低维材料可能是用于光催化方法的有前途的候选材料。在这里,我们使用了大量的第一性原理计算来研究二维碳氮化物的新制造成员的应用,包括tg -C 3 N 4,hg -C 3 N 4,C 2 N和C 3N用于水分解。演示了通过N型,P型和等电子掺杂剂(例如B,N,P,Si和Ge)进行能带工程,以调节电子结构,优化光吸收和光催化的能带排列。原始的tg -C 3 N 4,hg -C 3 N 4和C 2N晶体的带隙分别为3.190、2.772和2.465 eV,这不适合进行水分解。在这些掺杂剂中,Si和Ge掺杂剂可使碳氮化物的带隙变窄约0.5-1.0 eV,并在可见光谱中增加其光吸收。这项研究表明掺杂等电子元素的潜力,可以大大改善氮化碳纳米结构的光催化特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号