当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reagent controlled stereoselective synthesis of α-glucans
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-19 , DOI: 10.1021/jacs.8b00669 Liming Wang 1 , Herman S Overkleeft 1 , Gijsbert A van der Marel 1 , Jeroen D C Codée 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-19 , DOI: 10.1021/jacs.8b00669 Liming Wang 1 , Herman S Overkleeft 1 , Gijsbert A van der Marel 1 , Jeroen D C Codée 1
Affiliation
|
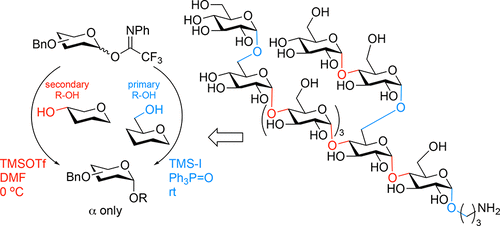
The development of a general glycosylation method that allows for the stereoselective construction of glycosidic linkages is a tremendous challenge. Because of the differences in steric and electronic properties of the building blocks used, the outcome of a glycosylation reaction can vary greatly when switching form one glycosyl donor–acceptor pair to another. We here report a strategy to install cis-glucosidic linkages in a fully stereoselective fashion that is under direct control of the reagents used to activate a single type of donor building block. The activating reagents are tuned to the intrinsic reactivity of the acceptor alcohol to match the reactivity of the glycosylating agent with the reactivity of the incoming nucleophile. A protecting group strategy is introduced that is based on the sole use of benzyl-ether type protecting groups to circumvent changes in reactivity as a result of the protecting groups. For the stereoselective construction of the α-glucosyl linkages to a secondary alcohol, a per-benzylated glusosyl imidate donor is activated with a combination of trimethylsilyltriflate and DMF, while activation of the same imidate donor with trimethylsilyl iodide in the presence of triphenylphosphine oxide allows for the stereoselective cis-glucosylation of primary alcohols. The effectiveness of the strategy is illustrated in the modular synthesis of a Mycobacterium tuberculosis nonasaccharide, composed of an α-(1–4)-oligoglucose backbone bearing different α-glucosyl branches.
中文翻译:

α-葡聚糖的试剂控制立体选择性合成
开发一种能够立体选择性构建糖苷键的通用糖基化方法是一个巨大的挑战。由于所用结构单元的空间和电子特性存在差异,当将一个糖基供体-受体对转换为另一对时,糖基化反应的结果可能会有很大差异。我们在这里报告了一种以完全立体选择性的方式安装顺式糖苷键的策略,该策略在用于激活单一类型供体结构单元的试剂的直接控制下。活化试剂根据受体醇的固有反应性进行调节,以使糖基化剂的反应性与引入的亲核试剂的反应性相匹配。引入了基于单独使用苄基醚型保护基团的保护基策略,以避免保护基团导致的反应性变化。为了立体选择性地构建与仲醇的 α-葡萄糖基键,用三氟甲磺酸三甲基硅酯和 DMF 的组合活化全苄基化的亚胺酸葡萄糖基供体,而在三苯基膦氧化物存在下用三甲基碘化硅活化相同的亚氨酸酯供体,从而允许伯醇的立体选择性顺式葡萄糖基化。该策略的有效性在结核分枝杆菌九糖的模块化合成中得到了证明,该九糖由带有不同 α-葡萄糖基分支的 α-(1-4)-低聚葡萄糖主链组成。
更新日期:2018-03-19
中文翻译:

α-葡聚糖的试剂控制立体选择性合成
开发一种能够立体选择性构建糖苷键的通用糖基化方法是一个巨大的挑战。由于所用结构单元的空间和电子特性存在差异,当将一个糖基供体-受体对转换为另一对时,糖基化反应的结果可能会有很大差异。我们在这里报告了一种以完全立体选择性的方式安装顺式糖苷键的策略,该策略在用于激活单一类型供体结构单元的试剂的直接控制下。活化试剂根据受体醇的固有反应性进行调节,以使糖基化剂的反应性与引入的亲核试剂的反应性相匹配。引入了基于单独使用苄基醚型保护基团的保护基策略,以避免保护基团导致的反应性变化。为了立体选择性地构建与仲醇的 α-葡萄糖基键,用三氟甲磺酸三甲基硅酯和 DMF 的组合活化全苄基化的亚胺酸葡萄糖基供体,而在三苯基膦氧化物存在下用三甲基碘化硅活化相同的亚氨酸酯供体,从而允许伯醇的立体选择性顺式葡萄糖基化。该策略的有效性在结核分枝杆菌九糖的模块化合成中得到了证明,该九糖由带有不同 α-葡萄糖基分支的 α-(1-4)-低聚葡萄糖主链组成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号