当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective reduction of the tricarbonyl moiety in bicyclic tetramates giving pyroglutamates†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8ob00187a Laia Josa-Culleré 1, 2, 3, 4, 5 , Kirsten E. Christensen 1, 2, 3, 4, 5 , Mark G. Moloney 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8ob00187a Laia Josa-Culleré 1, 2, 3, 4, 5 , Kirsten E. Christensen 1, 2, 3, 4, 5 , Mark G. Moloney 1, 2, 3, 4, 5
Affiliation
|
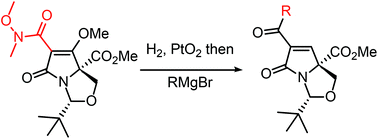
The reduction of C(6)-acyl bicyclic tetramic acids has been achieved with complete diastereoselectivity via catalytic hydrogenation using PtO2. The resulting pyroglutamates had potent antibacterial and anticancer properties and will allow the preparation of simple mimics of pyroglutamate-containing natural products.
中文翻译:

非对映选择性还原双环四酸酯中的三羰基部分,得到焦谷氨酸盐†
的还原Ç(6) -酰基双环特特拉姆酸已经用完整的非对映选择性实现通过使用PtO的催化氢化2。所得焦谷氨酸盐具有有效的抗菌和抗癌特性,可以制备含焦谷氨酸盐的天然产物的简单模拟物。
更新日期:2018-03-19
中文翻译:

非对映选择性还原双环四酸酯中的三羰基部分,得到焦谷氨酸盐†
的还原Ç(6) -酰基双环特特拉姆酸已经用完整的非对映选择性实现通过使用PtO的催化氢化2。所得焦谷氨酸盐具有有效的抗菌和抗癌特性,可以制备含焦谷氨酸盐的天然产物的简单模拟物。










































 京公网安备 11010802027423号
京公网安备 11010802027423号