当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Thermodynamic Modeling of N-(4-Methylphenyl-Z-3-chloro-2-(phenylthio)propenamide in 12 Pure Solvents at Temperatures Ranging from 278.15 to 318.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acs.jced.7b01011 Brian de Souza 1 , Leila Keshavarz 1 , René R. E. Steendam 1 , Olga C. Dennehy 2 , Denis Lynch 2 , Stuart G. Collins 2 , Humphrey A. Moynihan 2 , Anita R. Maguire 3 , Patrick J. Frawley 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acs.jced.7b01011 Brian de Souza 1 , Leila Keshavarz 1 , René R. E. Steendam 1 , Olga C. Dennehy 2 , Denis Lynch 2 , Stuart G. Collins 2 , Humphrey A. Moynihan 2 , Anita R. Maguire 3 , Patrick J. Frawley 1
Affiliation
|
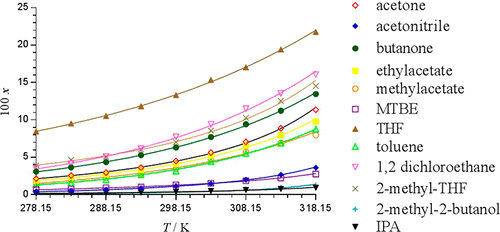
α-Thio-β-chloroacrylamides are of considerable synthetic utility due to their versatile reactivity profile enabling a diverse range of useful transformations. Availability of accurate and extensive solubility data and models is a prerequisite for advanced process optimization of such valuable pure synthetic intermediate compounds, in particular facilitating their isolation with a high degree of efficiency and control. As an illustrative example the solubility of one such derivative, N-(4-methylphenyl-Z-3-chloro-2-(phenylthio)propenamide (Z-1), is described in the present work. Solubility data is reported in 12 pure solvents specifically selected for their potential utility in synthesis and isolation at scale. Solubility data are determined using the gravimetric method across a range of temperatures T= (278.15 to 318.15) K under pressure of 0.1 MPa. On a molar basis, the solubility of Z-1 at temperature T = 298.15 K was observed to follow the order: tetrahydrofuran > 1,2-dichloroethane > 2-methyltetrahydrofuran > butanone > acetone > ethyl acetate > methyl acetate > toluene > tert-butyl methyl ether > acetonitrile > 2-propanol > 2-methyl-2-butanol. The experimental solubility data were correlated by the modified Apelblat, Margules, Van-Laar, Wilson, and nonrandom two-liquid (NRTL) models. The NRTL model was found to result in the lowest error for 8 of the 12 solvents tested. In the case of acetonitrile, the Wilson model had a slightly lower mean square error of 3.52 × 10–4 while for methyl acetate and 1,2-dichloroethane the Van-Laar model had the smallest mean square error of 1.47 × 10–3 and 3.54 × 10–4, respectively. The provision of solubility data and models for such a prized and versatile compound will assist with further development of continuous isolation strategies.
中文翻译:

N-(4-甲基苯基-Z -3-氯-2-(苯硫基)丙烯酰胺在12种纯溶剂中的溶解度测量和热力学模型,温度范围为278.15至318.15 K
由于α-硫代-β-氯丙烯酰胺具有广泛的反应活性,可实现多种有用的转化,因此具有相当大的合成用途。准确,广泛的溶解度数据和模型的可用性是此类有价值的纯合成中间体化合物进行高级工艺优化的先决条件,尤其是以高效率和可控制性促进它们的分离。作为说明性实例,一种这样的衍生物N-(4-甲基苯基-Z -3-氯-2-(苯硫基)丙烯酰胺(Z - 1),将在当前工作中进行介绍。报告了在12种纯溶剂中的溶解度数据,这些纯溶剂是根据其在大规模合成和分离中的潜在效用而专门选择的。使用重量分析法在0.1 MPa的压力下,在温度T =(278.15至318.15)K的范围内确定溶解度数据。以摩尔为基准,观察到Z - 1在温度T = 298.15 K时的溶解度遵循以下顺序:四氢呋喃> 1,2-二氯乙烷> 2-甲基四氢呋喃>丁酮>丙酮>乙酸乙酯>乙酸甲酯>甲苯>叔胺-丁基甲基醚>乙腈> 2-丙醇> 2-甲基-2-丁醇。实验溶解度数据通过修改后的Apelblat,Margules,Van-Laar,Wilson和非随机两液(NRTL)模型进行关联。发现NRTL模型导致所测试的12种溶剂中的8种的最低误差。对于乙腈,Wilson模型的均方误差略低,为3.52×10 -4,而对于乙酸甲酯和1,2-二氯乙烷,Van-Laar模型的均方误差最小,为1.47×10 –3,而分别为3.54×10 –4。为这种珍贵多功能的化合物提供溶解度数据和模型将有助于进一步开发连续分离策略。
更新日期:2018-03-20
中文翻译:

N-(4-甲基苯基-Z -3-氯-2-(苯硫基)丙烯酰胺在12种纯溶剂中的溶解度测量和热力学模型,温度范围为278.15至318.15 K
由于α-硫代-β-氯丙烯酰胺具有广泛的反应活性,可实现多种有用的转化,因此具有相当大的合成用途。准确,广泛的溶解度数据和模型的可用性是此类有价值的纯合成中间体化合物进行高级工艺优化的先决条件,尤其是以高效率和可控制性促进它们的分离。作为说明性实例,一种这样的衍生物N-(4-甲基苯基-Z -3-氯-2-(苯硫基)丙烯酰胺(Z - 1),将在当前工作中进行介绍。报告了在12种纯溶剂中的溶解度数据,这些纯溶剂是根据其在大规模合成和分离中的潜在效用而专门选择的。使用重量分析法在0.1 MPa的压力下,在温度T =(278.15至318.15)K的范围内确定溶解度数据。以摩尔为基准,观察到Z - 1在温度T = 298.15 K时的溶解度遵循以下顺序:四氢呋喃> 1,2-二氯乙烷> 2-甲基四氢呋喃>丁酮>丙酮>乙酸乙酯>乙酸甲酯>甲苯>叔胺-丁基甲基醚>乙腈> 2-丙醇> 2-甲基-2-丁醇。实验溶解度数据通过修改后的Apelblat,Margules,Van-Laar,Wilson和非随机两液(NRTL)模型进行关联。发现NRTL模型导致所测试的12种溶剂中的8种的最低误差。对于乙腈,Wilson模型的均方误差略低,为3.52×10 -4,而对于乙酸甲酯和1,2-二氯乙烷,Van-Laar模型的均方误差最小,为1.47×10 –3,而分别为3.54×10 –4。为这种珍贵多功能的化合物提供溶解度数据和模型将有助于进一步开发连续分离策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号