Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Association of Inhaled Corticosteroids and Long-Acting Muscarinic Antagonists With Asthma Control in Patients With Uncontrolled, Persistent Asthma
JAMA ( IF 63.1 ) Pub Date : 2018-04-10 , DOI: 10.1001/jama.2018.2757 Diana M. Sobieraj 1 , William L. Baker 1 , Elaine Nguyen 1 , Erin R. Weeda 1 , Craig I. Coleman 1 , C. Michael White 1 , Stephen C. Lazarus 2 , Kathryn V. Blake 3 , Jason E. Lang 4
JAMA ( IF 63.1 ) Pub Date : 2018-04-10 , DOI: 10.1001/jama.2018.2757 Diana M. Sobieraj 1 , William L. Baker 1 , Elaine Nguyen 1 , Erin R. Weeda 1 , Craig I. Coleman 1 , C. Michael White 1 , Stephen C. Lazarus 2 , Kathryn V. Blake 3 , Jason E. Lang 4
Affiliation
|
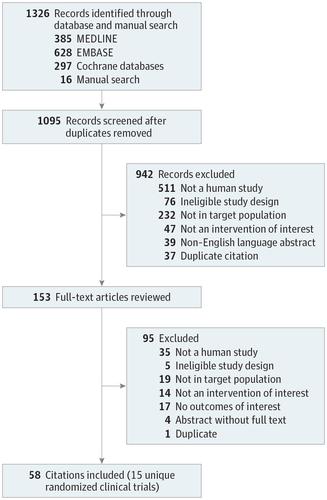
Importance Long-acting muscarinic antagonists (LAMAs) are a potential adjunct therapy to inhaled corticosteroids in the management of persistent asthma. Objective To conduct a systematic review and meta-analysis of the effects associated with LAMA vs placebo or vs other controllers as an add-on therapy to inhaled corticosteroids and the use of a LAMA as add-on therapy to inhaled corticosteroids and long-acting &bgr;-agonists (LABAs; hereafter referred to as triple therapy) vs inhaled corticosteroids and LABA in patients with uncontrolled, persistent asthma. Data Sources MEDLINE, EMBASE, Cochrane databases, and clinical trial registries (earliest date through November 28, 2017). Study Selection Two reviewers selected randomized clinical trials or observational studies evaluating a LAMA vs placebo or vs another controller as an add-on therapy to inhaled corticosteroids or triple therapy vs inhaled corticosteroids and LABA in patients with uncontrolled, persistent asthma reporting on an outcome of interest. Data Extraction and Synthesis Meta-analyses using a random-effects model was conducted to calculate risk ratios (RRs), risk differences (RDs), and mean differences (MDs) with corresponding 95% CIs. Citation screening, data abstraction, risk assessment, and strength-of-evidence grading were completed by 2 independent reviewers. Main Outcomes and Measures Asthma exacerbations. Results Of 1326 records identified, 15 randomized clinical trials (N = 7122 patients) were included. Most trials assessed adding LAMA vs placebo or LAMA vs LABA to inhaled corticosteroids. Adding LAMA vs placebo to inhaled corticosteroids was associated with a significantly reduced risk of exacerbation requiring systemic corticosteroids (RR, 0.67 [95% CI, 0.48 to 0.92]; RD, −0.02 [95% CI, −0.04 to 0.00]). Compared with adding LABA, adding LAMA to inhaled corticosteroids was not associated with significant improvements in exacerbation risk (RR, 0.87 [95% CI, 0.53 to 1.42]; RD, 0.00 [95% CI, −0.02 to 0.02]), or any other outcomes of interest. Triple therapy was not significantly associated with improved exacerbation risk vs inhaled corticosteroids and LABA (RR, 0.84 [95% CI, 0.57 to 1.22]; RD, −0.01 [95% CI, −0.08 to 0.07]). Conclusions and Relevance In this systematic review and meta-analysis, the use of LAMA compared with placebo as add-on therapy to inhaled corticosteroids was associated with a lower risk of asthma exacerbations; however, the association of LAMA with benefit may not be greater than that with LABA. Triple therapy was not associated with a lower risk of exacerbations.
中文翻译:

吸入性皮质类固醇和长效毒蕈碱拮抗剂与不受控制的持续性哮喘患者哮喘控制的关联
重要性 长效毒蕈碱拮抗剂 (LAMA) 是吸入性皮质类固醇治疗持续性哮喘的潜在辅助疗法。目的对与 LAMA 与安慰剂或与其他控制剂作为吸入性皮质类固醇的附加疗法以及使用 LAMA 作为吸入性皮质类固醇和长效 &bgr 的附加疗法相关的影响进行系统评价和荟萃分析;-激动剂(LABA;以下称为三联疗法)与吸入性皮质类固醇和 LABA 在不受控制的持续性哮喘患者中的比较。数据来源 MEDLINE、EMBASE、Cochrane 数据库和临床试验注册中心(最早日期至 2017 年 11 月 28 日)。研究选择 两名审查员选择了随机临床试验或观察性研究,评估 LAMA 与安慰剂或另一种控制剂作为吸入性皮质类固醇的附加疗法或三联疗法与吸入性皮质类固醇和 LABA 对未控制的持续性哮喘患者报告感兴趣的结果. 使用随机效应模型进行数据提取和综合 Meta 分析,以计算风险比 (RR)、风险差异 (RD) 和平均差异 (MD),并具有相应的 95% CI。引文筛选、数据提取、风险评估和证据强度分级由 2 名独立审查员完成。主要结果和措施 哮喘恶化。结果 在确定的 1326 条记录中,纳入了 15 项随机临床试验(N = 7122 名患者)。大多数试验评估了在吸入皮质类固醇中添加 LAMA 与安慰剂或 LAMA 与 LABA。在吸入性皮质类固醇中加入 LAMA 与安慰剂显着降低需要全身性皮质类固醇的恶化风险(RR,0.67 [95% CI,0.48 至 0.92];RD,-0.02 [95% CI,-0.04 至 0.00])。与添加 LABA 相比,在吸入糖皮质激素中添加 LAMA 与急性加重风险的显着改善无关(RR,0.87 [95% CI,0.53 至 1.42];RD,0.00 [95% CI,-0.02 至 0.02]),或任何其他感兴趣的结果。与吸入糖皮质激素和 LABA 相比,三联疗法与改善的恶化风险没有显着相关性(RR,0.84 [95% CI,0.57 至 1.22];RD,-0.01 [95% CI,-0.08 至 0.07])。结论和相关性 在本系统评价和荟萃分析中,与安慰剂相比,使用 LAMA 作为吸入性皮质类固醇的附加疗法与较低的哮喘恶化风险相关;然而,LAMA 与获益的相关性可能不会大于与 LABA 的相关性。三联疗法与较低的恶化风险无关。
更新日期:2018-04-10
中文翻译:

吸入性皮质类固醇和长效毒蕈碱拮抗剂与不受控制的持续性哮喘患者哮喘控制的关联
重要性 长效毒蕈碱拮抗剂 (LAMA) 是吸入性皮质类固醇治疗持续性哮喘的潜在辅助疗法。目的对与 LAMA 与安慰剂或与其他控制剂作为吸入性皮质类固醇的附加疗法以及使用 LAMA 作为吸入性皮质类固醇和长效 &bgr 的附加疗法相关的影响进行系统评价和荟萃分析;-激动剂(LABA;以下称为三联疗法)与吸入性皮质类固醇和 LABA 在不受控制的持续性哮喘患者中的比较。数据来源 MEDLINE、EMBASE、Cochrane 数据库和临床试验注册中心(最早日期至 2017 年 11 月 28 日)。研究选择 两名审查员选择了随机临床试验或观察性研究,评估 LAMA 与安慰剂或另一种控制剂作为吸入性皮质类固醇的附加疗法或三联疗法与吸入性皮质类固醇和 LABA 对未控制的持续性哮喘患者报告感兴趣的结果. 使用随机效应模型进行数据提取和综合 Meta 分析,以计算风险比 (RR)、风险差异 (RD) 和平均差异 (MD),并具有相应的 95% CI。引文筛选、数据提取、风险评估和证据强度分级由 2 名独立审查员完成。主要结果和措施 哮喘恶化。结果 在确定的 1326 条记录中,纳入了 15 项随机临床试验(N = 7122 名患者)。大多数试验评估了在吸入皮质类固醇中添加 LAMA 与安慰剂或 LAMA 与 LABA。在吸入性皮质类固醇中加入 LAMA 与安慰剂显着降低需要全身性皮质类固醇的恶化风险(RR,0.67 [95% CI,0.48 至 0.92];RD,-0.02 [95% CI,-0.04 至 0.00])。与添加 LABA 相比,在吸入糖皮质激素中添加 LAMA 与急性加重风险的显着改善无关(RR,0.87 [95% CI,0.53 至 1.42];RD,0.00 [95% CI,-0.02 至 0.02]),或任何其他感兴趣的结果。与吸入糖皮质激素和 LABA 相比,三联疗法与改善的恶化风险没有显着相关性(RR,0.84 [95% CI,0.57 至 1.22];RD,-0.01 [95% CI,-0.08 至 0.07])。结论和相关性 在本系统评价和荟萃分析中,与安慰剂相比,使用 LAMA 作为吸入性皮质类固醇的附加疗法与较低的哮喘恶化风险相关;然而,LAMA 与获益的相关性可能不会大于与 LABA 的相关性。三联疗法与较低的恶化风险无关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号