当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Acylselenourea–Diselenide Structures: New Potent and Selective Antitumoral Agents as Autophagy Activators
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00482 Pablo Garnica 1, 2 , Ignacio Encío 3 , Daniel Plano 1, 2 , Juan A. Palop 1, 2 , Carmen Sanmartín 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00482 Pablo Garnica 1, 2 , Ignacio Encío 3 , Daniel Plano 1, 2 , Juan A. Palop 1, 2 , Carmen Sanmartín 1, 2
Affiliation
|
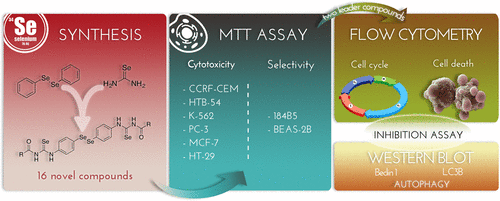
A series of 16 new diselenide–acylselenourea conjugates have been designed following the fragment-based drug strategy. Compound in vitro cytotoxic potential was evaluated against six human cancer cell lines and two nonmalignant derived cell lines with the aim of determining their potency and selectivity. Nine derivatives exhibited GI50 values under 10 μM in at least four cancer cell lines. A clear gap situated phenyl substitution over heterocyclic moieties in terms of selectivity. Among carbocyclic compounds, derivatives 2 and 7 significantly inhibited cell growth of breast adenocarcinoma cells with GI50 values of 1.30 and 0.15 nM, respectively, with selectivity indexes 12 and 121 times higher than those obtained for doxorubicin. Preliminary mechanistic studies indicated that compounds 2 and 7 induce cell cycle arrest and autophagy-dependent cell death evidenced by the blockage of cell death with pretreatment with wortmannin or chloroquine and confirmed by the upregulation of the markers Beclin1 and LC3B in MCF-7 cells.
中文翻译:

组合的酰基硒脲-二硒化物结构:新型有力和选择性抗肿瘤剂作为自噬激活剂。
按照基于片段的药物策略,已经设计了一系列16种新的二硒化物-酰基硒脲脲偶联物。针对六种人类癌细胞系和两种非恶性衍生细胞系,评估了化合物的体外细胞毒性潜力,旨在确定其效能和选择性。在至少四个癌细胞系中,有九种衍生物在10μM以下显示GI 50值。就选择性而言,明显的缺口位于苯基取代杂环部分上。在碳环化合物中,衍生物2和7具有GI 50显着抑制乳腺癌细胞的生长选择性分别为1.30和0.15 nM,选择性指数比对阿霉素的选择性指数高12和121倍。初步的机理研究表明,化合物2和7会诱导细胞周期停滞和自噬依赖性细胞死亡,这可以通过用渥曼青霉素或氯喹预处理阻止细胞死亡来证明,并通过MCF-7细胞中标志Beclin1和LC3B的上调来证实。
更新日期:2018-03-13
中文翻译:

组合的酰基硒脲-二硒化物结构:新型有力和选择性抗肿瘤剂作为自噬激活剂。
按照基于片段的药物策略,已经设计了一系列16种新的二硒化物-酰基硒脲脲偶联物。针对六种人类癌细胞系和两种非恶性衍生细胞系,评估了化合物的体外细胞毒性潜力,旨在确定其效能和选择性。在至少四个癌细胞系中,有九种衍生物在10μM以下显示GI 50值。就选择性而言,明显的缺口位于苯基取代杂环部分上。在碳环化合物中,衍生物2和7具有GI 50显着抑制乳腺癌细胞的生长选择性分别为1.30和0.15 nM,选择性指数比对阿霉素的选择性指数高12和121倍。初步的机理研究表明,化合物2和7会诱导细胞周期停滞和自噬依赖性细胞死亡,这可以通过用渥曼青霉素或氯喹预处理阻止细胞死亡来证明,并通过MCF-7细胞中标志Beclin1和LC3B的上调来证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号