当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Promiscuous activity of C-acyltransferase from Pseudomonas protegens: synthesis of acetanilides in aqueous buffer†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8cc00290h Anna Żądło-Dobrowolska 1, 2, 3, 4, 5 , Nina G. Schmidt 1, 2, 3, 4, 5 , Wolfgang Kroutil 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8cc00290h Anna Żądło-Dobrowolska 1, 2, 3, 4, 5 , Nina G. Schmidt 1, 2, 3, 4, 5 , Wolfgang Kroutil 1, 2, 3, 4, 5
Affiliation
|
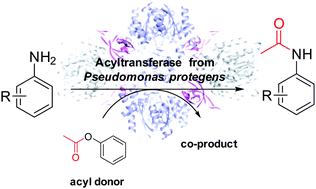
Amide bond formation has considerable significance in synthetic chemistry. Although the C-acyltransferase from Pseudomonas protegens has been found to catalyze C–C bond formation in nature as well as in in vitro experiments with non-natural substrates, it is now shown that the enzyme is also able to catalyze amide formation using aniline derivatives as substrates with promiscuous activity. Importantly, the amide formation was enabled in aqueous buffer. Identifying phenyl acetate as the most suitable acetyl donor, the products were obtained with up to >99% conversion and up to 99% isolated yield.
中文翻译:

蛋白假单胞菌C-酰基转移酶的混杂活性:在水缓冲液中对乙酰苯胺的合成†
酰胺键的形成在合成化学中具有相当重要的意义。尽管在自然界以及在非天然底物的体外实验中已发现假单胞菌蛋白的C-酰基转移酶可催化C-C键的形成,但现在表明该酶也能够使用苯胺衍生物催化酰胺的形成。作为具有混杂活性的底物。重要的是,能够在水性缓冲液中形成酰胺。确定乙酸苯酯为最合适的乙酰基供体,获得的产物具有最高> 99%的转化率和最高99%的分离产率。
更新日期:2018-03-19
中文翻译:

蛋白假单胞菌C-酰基转移酶的混杂活性:在水缓冲液中对乙酰苯胺的合成†
酰胺键的形成在合成化学中具有相当重要的意义。尽管在自然界以及在非天然底物的体外实验中已发现假单胞菌蛋白的C-酰基转移酶可催化C-C键的形成,但现在表明该酶也能够使用苯胺衍生物催化酰胺的形成。作为具有混杂活性的底物。重要的是,能够在水性缓冲液中形成酰胺。确定乙酸苯酯为最合适的乙酰基供体,获得的产物具有最高> 99%的转化率和最高99%的分离产率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号