当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction†
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8sc00604k Panpan Su 1, 2, 3, 4 , Kazuyuki Iwase 4, 5, 6, 7 , Takashi Harada 1, 2, 3, 4 , Kazuhide Kamiya 1, 2, 3, 4, 8 , Shuji Nakanishi 1, 2, 3, 4, 8
Chemical Science ( IF 8.4 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8sc00604k Panpan Su 1, 2, 3, 4 , Kazuyuki Iwase 4, 5, 6, 7 , Takashi Harada 1, 2, 3, 4 , Kazuhide Kamiya 1, 2, 3, 4, 8 , Shuji Nakanishi 1, 2, 3, 4, 8
Affiliation
|
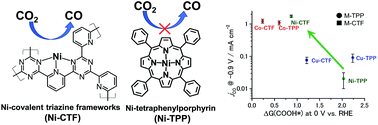
The electrochemical reduction of carbon dioxide (CO2) has attracted considerable attention as a means of maintaining the carbon cycle. This process still suffers from poor performance, including low faradaic efficiencies and high overpotential. Herein, we attempted to use coordination number as a control parameter to improve the electrocatalytic performance of metal species that have previously been thought to have no CO2 reduction activity. Covalent triazine frameworks (CTF) modified with coordinatively-unsaturated 3d metal atoms (Co, Ni or Cu) were developed for efficient electroreduction of CO2. Co-CTF and Ni-CTF materials effectively reduced CO2 to CO from −0.5 V versus RHE. The faradaic efficiency of the Ni-CTF during CO formation reached 90% at −0.8 V versus RHE. The performance of Ni-CTF is much higher than that of the corresponding metal-porphyrin (using tetraphenylporphyrin; TPP). First principles calculations demonstrated that the intermediate species (adsorbed COOH) was stabilized on the metal atoms in the CTF due to the low-coordination structure of this support. Thus, the free energy barriers for the formation of adsorbed COOH on the metal atoms in the CTF supports were lower than those on the TPP supports.
中文翻译:

配位不饱和的Co或Ni原子修饰的共价三嗪骨架,用于CO 2电化学还原†
作为维持碳循环的手段,二氧化碳(CO 2)的电化学还原引起了相当大的关注。该过程仍然遭受性能不佳的困扰,包括法拉第效率低和过电位高。在本文中,我们试图使用配位数作为控制参数来改善先前被认为不具有CO 2还原活性的金属物质的电催化性能。为了有效地电还原CO 2,开发了用不饱和3d金属原子(Co,Ni或Cu)修饰的共价三嗪骨架(CTF)。共CTF和Ni-CTF材料有效地减少CO 2从CO到-0.5V的对比RHE。与RHE相比,在-0.8 V时,CO形成过程中Ni-CTF的法拉第效率达到了90%。Ni-CTF的性能远远高于相应的金属卟啉(使用四苯基卟啉; TPP)。第一原理计算表明,由于该载体的低配位结构,中间物种(吸附的COOH)稳定在CTF中的金属原子上。因此,用于在CTF载体中的金属原子上形成吸附的COOH的自由能垒比在TPP载体上的自由能垒低。
更新日期:2018-03-19
中文翻译:

配位不饱和的Co或Ni原子修饰的共价三嗪骨架,用于CO 2电化学还原†
作为维持碳循环的手段,二氧化碳(CO 2)的电化学还原引起了相当大的关注。该过程仍然遭受性能不佳的困扰,包括法拉第效率低和过电位高。在本文中,我们试图使用配位数作为控制参数来改善先前被认为不具有CO 2还原活性的金属物质的电催化性能。为了有效地电还原CO 2,开发了用不饱和3d金属原子(Co,Ni或Cu)修饰的共价三嗪骨架(CTF)。共CTF和Ni-CTF材料有效地减少CO 2从CO到-0.5V的对比RHE。与RHE相比,在-0.8 V时,CO形成过程中Ni-CTF的法拉第效率达到了90%。Ni-CTF的性能远远高于相应的金属卟啉(使用四苯基卟啉; TPP)。第一原理计算表明,由于该载体的低配位结构,中间物种(吸附的COOH)稳定在CTF中的金属原子上。因此,用于在CTF载体中的金属原子上形成吸附的COOH的自由能垒比在TPP载体上的自由能垒低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号