当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cooperative role of halogen and hydrogen bonding in the stabilization of water adducts with apolar molecules†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8nj00552d Matteo De Santis 1, 2, 3, 4 , Francesca Nunzi 1, 2, 3, 4, 5 , Diego Cesario 1, 2, 3, 4 , Leonardo Belpassi 3, 3, 4, 5, 6 , Francesco Tarantelli 1, 2, 3, 4, 5 , David Cappelletti 1, 2, 3, 4 , Fernando Pirani 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c8nj00552d Matteo De Santis 1, 2, 3, 4 , Francesca Nunzi 1, 2, 3, 4, 5 , Diego Cesario 1, 2, 3, 4 , Leonardo Belpassi 3, 3, 4, 5, 6 , Francesco Tarantelli 1, 2, 3, 4, 5 , David Cappelletti 1, 2, 3, 4 , Fernando Pirani 1, 2, 3, 4
Affiliation
|
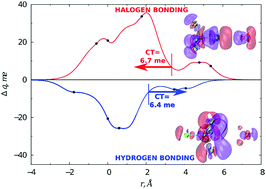
The relative role of halogen and hydrogen bonding in the stabilization of water adducts with apolar molecules is investigated by an integrated experimental–theoretical study. Molecular beam scattering experiments led to the characterization of the gas phase behaviour of H2O–CCl4 and H2O–CF4 systems, with the determination of the absolute scale of the interaction. Highly accurate ab initio calculations allow identifying the most stable energy configurations and the electron charge transferred upon adduct formation. The combined study is important to unambiguously identify the role of various components in the interaction (electrostatic, dispersion, induction and charge transfer) and for formulating a reliable model of the interaction potential. Our results suggest that, while the water–CF4 adduct is basically bound through the balance between size (Pauli) repulsion and dispersion attraction, an appreciable intermolecular bond stabilization by charge transfer is operative in water–CCl4. We identified a sizable charge transfer not only from H2O to CCl4, mostly for the vertex (halogen bonding) configuration, but also from CCl4 to H2O, for configurations where an H atom points at a chlorine centre, thus evidencing a hydrogen bond.
中文翻译:

卤素和氢键在稳定具有非极性分子的水加合物中的协同作用†
通过综合的实验理论研究,研究了卤素和氢键在水与非极性分子加合物稳定中的相对作用。分子束散射实验导致了H 2 O–CCl 4和H 2 O–CF 4系统的气相行为表征,并确定了相互作用的绝对尺度。高度准确的从头算通过计算可以确定最稳定的能量构型以及加合物形成时转移的电子电荷。结合起来的研究对于清楚地确定各种组分在相互作用中的作用(静电,分散,感应和电荷转移)以及建立相互作用势的可靠模型非常重要。我们的结果表明,虽然水–CF 4加合物基本上通过大小(保利)排斥力和分散吸引之间的平衡而束缚,但在水–CCl 4中可通过电荷转移实现明显的分子间键稳定作用。我们确定了不仅从H 2 O到CCl 4的大量电荷转移,主要用于顶点(卤素键)构型,但也适用于从CCl 4到H 2 O的构型,其中H原子指向氯中心,从而证明存在氢键。
更新日期:2018-03-19
中文翻译:

卤素和氢键在稳定具有非极性分子的水加合物中的协同作用†
通过综合的实验理论研究,研究了卤素和氢键在水与非极性分子加合物稳定中的相对作用。分子束散射实验导致了H 2 O–CCl 4和H 2 O–CF 4系统的气相行为表征,并确定了相互作用的绝对尺度。高度准确的从头算通过计算可以确定最稳定的能量构型以及加合物形成时转移的电子电荷。结合起来的研究对于清楚地确定各种组分在相互作用中的作用(静电,分散,感应和电荷转移)以及建立相互作用势的可靠模型非常重要。我们的结果表明,虽然水–CF 4加合物基本上通过大小(保利)排斥力和分散吸引之间的平衡而束缚,但在水–CCl 4中可通过电荷转移实现明显的分子间键稳定作用。我们确定了不仅从H 2 O到CCl 4的大量电荷转移,主要用于顶点(卤素键)构型,但也适用于从CCl 4到H 2 O的构型,其中H原子指向氯中心,从而证明存在氢键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号