当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Following Ramachandran 2: exit vector plot (EVP) analysis of disubstituted saturated rings
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c7nj05015a Oleksandr O. Grygorenko 1, 2, 3, 4, 5 , Daryna Demenko 1, 2, 3 , Dmitry M. Volochnyuk 1, 2, 3, 6, 7 , Igor V. Komarov 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1039/c7nj05015a Oleksandr O. Grygorenko 1, 2, 3, 4, 5 , Daryna Demenko 1, 2, 3 , Dmitry M. Volochnyuk 1, 2, 3, 6, 7 , Igor V. Komarov 1, 2, 3, 4, 5
Affiliation
|
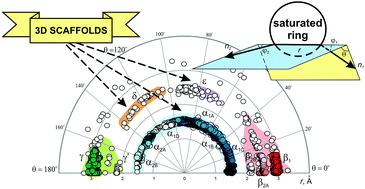
Analysis of disubstituted heteroaliphatic, as well as all common saturated rings using the exit vector plots (EVPs) tool is described, which is based on the Cambridge Structural Database (CSD) data. It is shown that the combined EVPs for saturated rings are similar to those observed for cycloalkanes (i.e. α, β, γ, and δ regions are found). In addition to that, a new region (ε) is found which is not accessible by simple cycloalkane derivatives. It is shown that while introducing saturated rings into the molecules is widely considered as an approach to increase their non-planarity, not many of them are truly three-dimensional; some recommendations are given to address this issue.
中文翻译:

继Ramachandran 2:双取代饱和环的出口向量图(EVP)分析
描述了基于剑桥结构数据库(CSD)数据的出口矢量绘图(EVP)工具对双取代杂脂族化合物以及所有常见饱和环的分析。结果表明,饱和环的合并EVP与环烷烃相似(即,发现有α,β,γ和δ区域)。除此之外,发现了新的区域(ε),简单的环烷烃衍生物无法进入该区域。结果表明,虽然将饱和环引入分子中被广泛认为是增加其非平面性的一种方法,但它们中的许多并不是真正的三维。提供了一些建议来解决此问题。
更新日期:2018-03-19
中文翻译:

继Ramachandran 2:双取代饱和环的出口向量图(EVP)分析
描述了基于剑桥结构数据库(CSD)数据的出口矢量绘图(EVP)工具对双取代杂脂族化合物以及所有常见饱和环的分析。结果表明,饱和环的合并EVP与环烷烃相似(即,发现有α,β,γ和δ区域)。除此之外,发现了新的区域(ε),简单的环烷烃衍生物无法进入该区域。结果表明,虽然将饱和环引入分子中被广泛认为是增加其非平面性的一种方法,但它们中的许多并不是真正的三维。提供了一些建议来解决此问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号