当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Innovative Application of Acid Leaching to Regenerate Li(Ni1/3Co1/3Mn1/3)O2 Cathodes from Spent Lithium-Ion Batteries
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acssuschemeng.7b04373 Xiaoxiao Zhang 1 , Yifan Bian 1 , Siwenyu Xu 1 , Ersha Fan 1 , Qing Xue 1 , Yibiao Guan 2 , Feng Wu 1, 3 , Li Li 1, 3 , Renjie Chen 1, 3
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acssuschemeng.7b04373 Xiaoxiao Zhang 1 , Yifan Bian 1 , Siwenyu Xu 1 , Ersha Fan 1 , Qing Xue 1 , Yibiao Guan 2 , Feng Wu 1, 3 , Li Li 1, 3 , Renjie Chen 1, 3
Affiliation
|
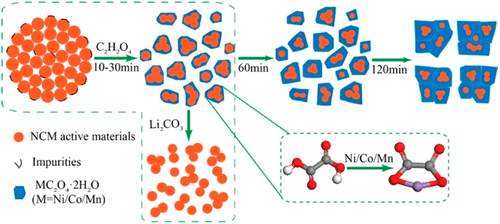
Rapid development of energy storage system causes a burst demand of lithium-ion batteries (LIBs), and large number of spent LIBs with high valuable metals are produced. Here we propose a novel application of oxalic acid leaching to regenerate Li(Ni1/3Co1/3Mn1/3)O2 (NCM) cathodes from spent LIBs. With lithium dissolving into the solution, the transition metals transform into oxalate precipitates and deposit on the surface of spent NCM cathodes, separating lithium and transition metals in one simple step. After mixing with certain amount of Li2CO3, the oxalate precipitates together with unreacted NCM are directly calcined into new NCM cathodes. The regenerated NCM after 10 min leaching exhibits the best electrochemical performances, delivering the highest initial specific discharge capacity of 168 mA h g–1 at 0.2C and 153.7 mA h g–1 after 150 cycles with a high capacity retention of 91.5%. The excellent electrochemical performances are attributed to the submicrometer particles and voids after calcination, as well as the optimal proportion of elements. This process can make the most of valuable metals in the spent cathodes, with >98.5% Ni, Co, and Mn recycled. It is simple and effective, and provides a novel perspective of recycling cathodes from spent LIBs.
中文翻译:

酸浸在废锂离子电池再生Li(Ni 1/3 Co 1/3 Mn 1/3)O 2阴极中的创新应用
储能系统的飞速发展导致对锂离子电池(LIB)的需求激增,并且产生了大量的废旧的LIB,其中包含高价金属。在这里,我们提出了一种草酸浸出的新应用,可以从用过的LIB再生Li(Ni 1/3 Co 1/3 Mn 1/3)O 2(NCM)阴极。随着锂溶解到溶液中,过渡金属转化为草酸盐沉淀并沉积在用过的NCM阴极表面上,只需一个简单的步骤即可分离锂和过渡金属。与一定量的Li 2 CO 3混合后,草酸盐沉淀与未反应的NCM一起直接煅烧成新的NCM阴极。浸出10分钟后再生的NCM表现出最佳的电化学性能,在0.2C下提供最高的初始比放电容量为168 mA hg –1,在150个循环后提供153.7 mA hg –1的最高放电容量,保持率高达91.5%。优异的电化学性能归因于煅烧后的亚微米级颗粒和空隙以及元素的最佳比例。此过程可以使废阴极中的有价值的金属得到最大利用,镍,钴和锰的回收率> 98.5%。它简单有效,为从废LIB中回收阴极提供了新颖的视角。
更新日期:2018-03-19
中文翻译:

酸浸在废锂离子电池再生Li(Ni 1/3 Co 1/3 Mn 1/3)O 2阴极中的创新应用
储能系统的飞速发展导致对锂离子电池(LIB)的需求激增,并且产生了大量的废旧的LIB,其中包含高价金属。在这里,我们提出了一种草酸浸出的新应用,可以从用过的LIB再生Li(Ni 1/3 Co 1/3 Mn 1/3)O 2(NCM)阴极。随着锂溶解到溶液中,过渡金属转化为草酸盐沉淀并沉积在用过的NCM阴极表面上,只需一个简单的步骤即可分离锂和过渡金属。与一定量的Li 2 CO 3混合后,草酸盐沉淀与未反应的NCM一起直接煅烧成新的NCM阴极。浸出10分钟后再生的NCM表现出最佳的电化学性能,在0.2C下提供最高的初始比放电容量为168 mA hg –1,在150个循环后提供153.7 mA hg –1的最高放电容量,保持率高达91.5%。优异的电化学性能归因于煅烧后的亚微米级颗粒和空隙以及元素的最佳比例。此过程可以使废阴极中的有价值的金属得到最大利用,镍,钴和锰的回收率> 98.5%。它简单有效,为从废LIB中回收阴极提供了新颖的视角。











































 京公网安备 11010802027423号
京公网安备 11010802027423号