当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Baylis-Hillman acetates in organic synthesis: A simple two-step strategy for oxindole-spiro-α-arylidene-γ-butyrolactone framework
Tetrahedron ( IF 2.1 ) Pub Date : 2018-03-17 , DOI: 10.1016/j.tet.2018.03.035 Deevi Basavaiah , Harathi Lingam , Thelagathoti Hari Babu
中文翻译:

有机合成中的Baylis-Hillman乙酸酯:羟吲哚-螺-α-亚芳基-γ-丁内酯框架的简单两步策略
更新日期:2018-03-17
Tetrahedron ( IF 2.1 ) Pub Date : 2018-03-17 , DOI: 10.1016/j.tet.2018.03.035 Deevi Basavaiah , Harathi Lingam , Thelagathoti Hari Babu
|
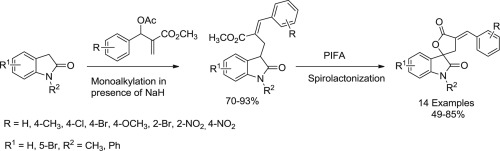
A facile, convenient, and two step strategy for synthesis of spirooxindoles containing α-arylidene-γ-butyrolactone moiety has been developed via monoalkylation of 2-oxindoles with Baylis-Hillman acetates followed by the spirolactonization of the resulting cinnamic esters on treatment with phenyliodine (III) bistrifluoroacetate (PIFA).
中文翻译:

有机合成中的Baylis-Hillman乙酸酯:羟吲哚-螺-α-亚芳基-γ-丁内酯框架的简单两步策略
通过将2-氧吲哚与Baylis-Hillman乙酸酯进行单烷基化,然后将生成的肉桂酸酯经苯碘化处理进行螺内酯化,已经开发了一种简便,方便且分两步的方法,用于合成含有α-亚芳基-γ-丁内酯部分的螺硫辛多酯。 III)双三氟乙酸盐(PIFA)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号