Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-03-16 , DOI: 10.1016/j.bmcl.2018.03.038 Fidelia Ijeoma Uche , James McCullagh , Timothy W.D. Claridge , Alan Richardson , Wen-Wu Li
|
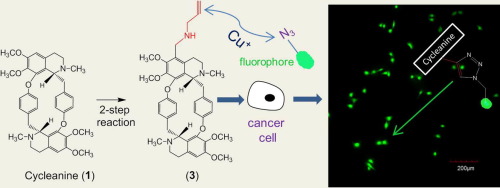
Our previous studies demonstrated that cycleanine, a macrocyclic bisbenzylisoquinoline (BBIQ) alkaloid, showed potent anti-ovarian cancer activity via apoptosis induction. Here, we synthesized two novel (aminoalkyl)cycleanine analogues (2 and 3) through a simple and efficient two-step reaction starting from cycleanine isolated from Triclisia subcordata Oliv. These analogues showed greater potency than the unmodified cycleanine in three human ovarian cancer cell lines. Both 2 and 3 induced apoptosis in ovarian cancer cells by activations of caspases 3/7, cleavage of PARP, increase in subG1 cell cycle phase and in the percentage of apoptotic cells. Further confocal fluorescence microscopy analysis confirmed the cellular uptake of alkaloids in ovarian cancer cells by using the unique (alkynyl)cycleanine (3) via click chemistry reaction. Our results suggest that cycleanine could be a hit compound for the future development in attacking ovarian cancer.
中文翻译:

(氨基烷基)环己胺类似物的合成:卵巢癌细胞的细胞毒性,细胞摄取和凋亡诱导
我们以前的研究表明,大环双苄基异喹啉(BBIQ)生物碱Cyclanine通过凋亡诱导显示出有效的抗卵巢癌活性。在这里,我们从简单和有效的两步反应开始,合成了两个新颖的(氨基烷基)环己胺类似物(2和3),该反应从分离自Triclisia subcordata Oliv的环烷胺开始。这些类似物在三种人卵巢癌细胞系中显示出比未修饰的环己氨酸更大的效价。2和3均通过胱天蛋白酶3/7的激活,PARP的裂解,subG 1的增加诱导卵巢癌细胞凋亡。细胞周期阶段和凋亡细胞的百分比。进一步的共聚焦荧光显微镜分析证实了通过点击化学反应使用独特的(炔基)Cyclanine(3),卵巢癌细胞中生物碱的细胞摄取。我们的结果表明,Cyclanine可能是攻击卵巢癌未来发展的重要化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号